26th Annual Glaucoma ReportCheck out the other feature articles in this month's report: |
Open-angle glaucoma (OAG) is a chronic and visually devastating disease with minimal symptoms until it reaches the advanced stage. The goal throughout treatment is to stave off progression and ensure a lifetime of preserved vision.1
But once progression is detected, the practitioner is faced with a challenging decision: re-educate the patient on the current regimen to boost medication adherence or change the treatment course. Thoroughly educating patients about the progressive nature of glaucoma and its treatments can help patients understand the importance of medication compliance.
If compliance is not the issue, clinicians should ensure the patient is using proper drop instillation techniques, as some patients may struggle with dexterity.
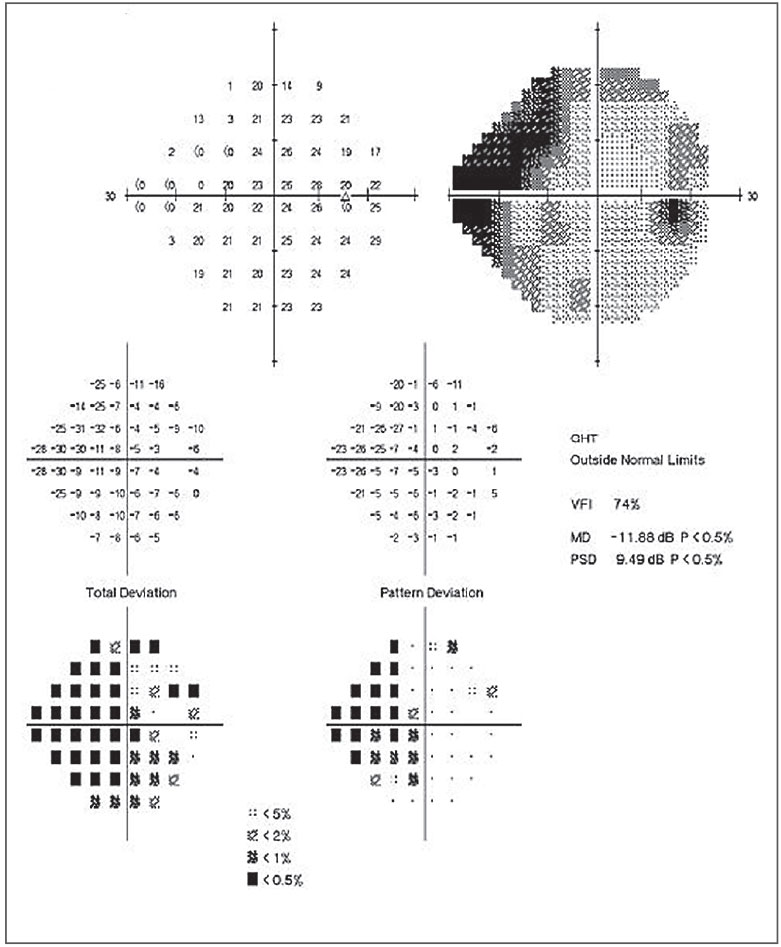 |
Fig. 1. This patient’s 24-2 field shows moderate visual field damage with central involvement. Click to enlarge image. |
Once clinicians address these issues, they can then reconsider the efficacy of the medication regimen prescribed.
Before considering a change in the current management, clinicians must evaluate the risk factor profile for progression, target intraocular pressures (IOPs), and medication adherence and burden. Other important considerations include the potential benefits and risks of surgery and the risks of functional vision impairment if left untreated vs. age-related decline.2 This article discusses six considerations to help clinicians navigate the complicated management decisions necessary once a patient shows signs of glaucoma progression.
1. Assess Risk Factors
Unfortunately, disease progression isn’t always cut-and-dry. Many patients progress slowly with little impact on their vision while others progress rapidly with devastating consequences.1 To help detect rapid and severe progression, clinicians should perform three visual fields in the first year (i.e., at diagnosis, six months and 12 months); in year two, patients should have one visual field every six months. If the examination rules out rapid progression (i.e., greater than 0.5dB/year on mean deviation or pattern standard deviation), clinicians can scale back to one visual field per year if the patient remains stable (i.e., 0.1dB/year).
Thus, one of the most important factors in advancing glaucoma is the rate of progression. Ancillary testing with optical coherence tomography (OCT) and visual fields can help clinicians document structural and functional changes associated with progression (Tables 1-3).
Once progression is determined, clinicians must consider the patient’s age, general health status, life expectancy and expected rate of decline with current treatment to design the best adjunctive therapeutic approach based on each patient’s risk of visual decline.1,2 For example, patients aged 70 or older with slowly progressing glaucoma likely require less intense treatment or sometimes no additional treatment at all, while young glaucoma patients with fast progressing disease require quick action, an aggressive approach and possibly surgery.
Certain optic disc features can indicate a higher risk of visual decline in glaucoma patients. These include an increasing vertical cup-to-disc ratio with preferential rim loss to the inferior, inferotemporal, supratemporal and superior regions, the presence of Drance hemorrhages, increasing size of the parapapillary beta zone and new localized retinal nerve fiber layer (RNFL) defects.3
Other important clinical features putting the patient at risk for further disease progression include severe staging at the time of diagnosis, type of glaucoma (i.e., pseudoexfoliation, pigment dispersion) and large mean deviation (<-12.00dB) on perimetry. Higher peak and average IOPs at baseline, higher mean IOP or large IOP variation also put the patient at a higher risk for visual decline.1,2
2. Be Wary of Target IOPs
Studies show each 1mm Hg of increased IOP is associated with a 10% to 19% increased risk of progression.4 The best IOP for each patient isn’t necessarily a static number—it’s a balance between the risk of decreased vision related quality of life due to glaucoma and the risks of treatment.5 While insufficient evidence shows setting target IOPs is associated with better clinical outcomes, clinicians must consider the risks and benefits before establishing a target IOP for each patient.5 Considerations include short and long-term IOP fluctuations, inter-observer variability, patient life expectancy and treatment adherence.
Table 1. OCT RNFL Progression19-25
|
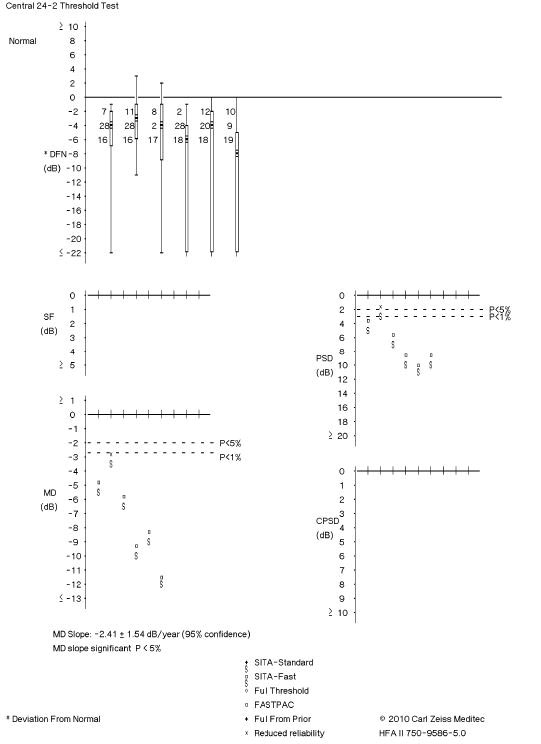 |
Fig. 2. This visual field readout shows trend-based change, which indicates visual field progression. Click to enlarge image. |
When resetting target IOPs after adjusting a patient’s glaucoma regimen, clinicians must evaluate the amount of glaucomatous damage, the average range of IOPs at which glaucomatous damage is occurring and the status of the fellow eye.5
Researchers suggests target IOPs may be particularly useful for patients at high risk of substantial vision loss and blindness.5 For those with low risk for visual loss, clinicians may do better focusing on reducing treatment side effects rather than achieving a particular IOP.5
To complicate matters further, a patient’s target IOP will likely change over time, especially if they experience accelerated progression with the current target or if the fellow eye’s visual status becomes significantly reduced.4
Target IOPs are useful broad guidelines in OAG therapy but should not be used in isolation from other information. Serial ancillary testing can help clinicians highlight progression and modify therapeutic measures when indicated.
With an appropriate IOP target range and continuous reassessment, glaucoma progression can be considerably slowed to reduce the probability of decreased vision-related quality of life.4
3. Set New Baselines
Once new therapy is initiated, clinicians must establish new baselines for perimetry, OCT RNFL and ganglion cell analysis (GCA), and photo documentation. The practitioner does not need to perform additional tests when setting these new baseline parameters. Instead, they can reference the last two tests performed to set a new baseline.1,2 Furthermore, guided progression analysis will support the analysis for trend-based change with these tests.
4. Consider SLT
Selective laser trabeculoplasty (SLT) was approved by the FDA in 2001 and has since proven itself an effective method for lowering IOP.6 SLT is often considered in cases of inadequate IOP reduction with medications, intolerance, allergy or poor adherence to medications (e.g., due to cost, cognitive decline, insufficient dexterity or tremor) and may be recommended at various points in the treatment arc, including as the initial treatment option.
Currently, SLT is less commonly offered as first-line therapy compared with topical medications for ocular hypertension (OHTN) or OAG. Recent evidence suggests SLT should be considered as a safe, effective alternative to medication as a primary therapy for a large subset of these patients.7,8
The Laser in Glaucoma and Ocular Hypertension Trial (LiGHT) Study Group found SLT could successfully arrest progression in 74% of patients with OHTN and newly diagnosed OAG for a period of at least three years without medications—a finding that should encourage providers to consider SLT as first-line therapy.7
Clinicians must consider many factors before recommending SLT to a patient, but they have fewer factors to consider if the goal is an attempt to eliminate glaucoma medication burden. It is well-established that a high baseline IOP positively correlates with the conventional measure of SLT success of ≥20% IOP reduction.9
The LiGHT Trial shows us that patients with OHTN and mild OAG are the most likely cohorts to achieve drop-free disease control at three years.6 Patients with more advanced disease or lower baseline IOP can still benefit from SLT but may need adjunctive medical therapy; however, it is likely fewer drops will be necessary—relatively sparing the ocular surface and potentially improving the patient’s medication adherence.7,8
Shedding LiGHT on SLTThe LiGHT Study Group conducted a large, prospective, randomized controlled trial with 718 patients (1,235 eyes) to compare standardized 360° SLT with eye drops in treatment-naïve patients. The majority of patients in each treatment arm were diagnosed with either OHTN or mild OAG—approximately 30% and 50%, respectively. Prostaglandin analogs were offered as the primary topical agent followed by adjunctive therapy with β-blockers, then carbonic anhydrase inhibitors or α-agonists. The patients were monitored for three years.7 SLT was not associated with any serious adverse events, but approximately one-third of patients experienced transient effects such as discomfort, blur, photophobia and ocular hyperemia. The SLT-first group experienced fewer drop-related side effects (5.7%) compared with the medication-first arm (20.2%), likely secondary to the reduction in the mean number of drops necessary in the former group.7 This is consistent with reports from pooled analyses comparing SLT with eye drops for OAG, including data from the LiGHT Study Group, demonstrating that SLT is effective at significantly reducing the number of topical medications necessary for adequate IOP control.8 The percentage of visits at target IOP was slightly higher for the SLT group when compared with the medication-first group: 93% vs. 91.3%. Fewer treatment escalations occurred in the SLT-first arm, none of which led to trabeculectomy compared with 11 eyes in the medication-first group. Ultimately, 74% of patients treated with SLT first were stable at three years without using any topical therapy. A second SLT was necessary in 25.7% of eyes. There were no significant differences in visual acuity, IOP or mean deviation loss on visual field testing between the two groups at the study’s conclusion.7 The LiGHT Study Group did not report data on medication adherence or persistence, which can significantly impact treatment escalations and outcomes. Studies show as few as 33% to 39% of patients persist with the initially prescribed medication at one year.28 The LiGHT trial design is clinically relevant due to its individualized treatment approach in which patients with more severe disease were assigned a lower initial target IOP with modifications made according to widely accepted and implemented clinical guidelines. Furthermore, it measured SLT success as controlling progression of neuropathy, rather than a percentage of IOP reduction. By stratifying these data based on disease stage, it showed a single SLT was far more likely to result in a controlled status without drops at three years in patients having either OHTN (72.8%) or mild OAG (64.3%) when compared with eyes with moderate (33.3%) and severe (9.6%) OAG.7 That is not to say SLT was ineffective in lowering IOP in more advanced stages. The mean treatment effect was similar among all stages (approximately 8mm Hg). It more likely reflects a standalone inability to meet the more stringent IOP goals newly diagnosed advanced disease warrants.6 In another study, 180° SLT was successful in 50% of eyes with advanced OAG when measured against the criteria of 30% IOP reduction from pre-treatment value and <18mm Hg.29 However, randomized controlled trial studies comparing SLT with medication-only treatment groups largely include milder cases of glaucoma, so further research is necessary to elucidate the role of SLT in advanced cases; for now, filtration surgery remains the standard in the context of progressive neuropathy.8,29 |
5. Do Your MIGS Research
In particular circumstances, minimally invasive glaucoma surgery (MIGS) may be a good option for mild to moderate glaucoma patients undergoing cataract surgery. A recent study shows 22% of cataract surgeries performed by glaucoma specialists in 2016 included a MIGS procedure.10 Numerous MIGS procedures exist, and they are minimally traumatic to the surrounding tissue and exhibit minimal tissue disruption. The various safety profiles are excellent compared with incisional surgery and glaucoma drainage device implantation.11-13 Wound healing is rapid with good preservation of vision.11-13
Table 2. OCT GCA Progression19-25
|
Furthermore, MIGS are combined with cataract surgery, and efficacy shows moderate to high IOP-lowering capabilities. One meta-analysis shows a decrease in IOP and a reduction in glaucoma medications after MIGS surgery with low complication rates.14
This therapeutic option could allow a significant number of OAG patients to reduce their medication burden with a lower risk of complications.3,14
6. Prepare for the Last Defense
Medical therapy is effective for the majority of glaucoma patients, but surgical means are recommended when patients experience fast rates of functional and/or structural progression, central visual field loss, suboptimal hypotensive IOP control with medical therapy and SLT, or they have uncontrolled moderate-severe disease.15
Incisional glaucoma filtration surgery includes trabeculectomy and glaucoma drainage devices such as the Ahmed glaucoma valve or Baerveldt glaucoma implant.11
Despite an increasing safety profile over the years, these techniques have higher postoperative risks compared with nonincisional surgeries, such as late-onset bleb infection and endophthalmitis, hypotony maculopathy, choroidal effusion or hemorrhage, flat anterior chamber, corneal damage and cataract.11
Because of these risks, incisional surgery is reserved for those with rapidly progressing glaucoma regardless of stage, those with severe glaucoma who failed with medical and noninvasive therapies and those with risk of visual impairment due to progressing central visual field loss.16,17 Furthermore, incisional surgery requires intense postoperative healing, wound modulation and strict follow-up exams to manage postoperative complications.
There can be more disadvantages than benefits in the mild to moderate glaucoma patient or for patients who want to reduce the medication burden because therapeutic efficacy can gradually decrease over time, requiring repeat surgery.17
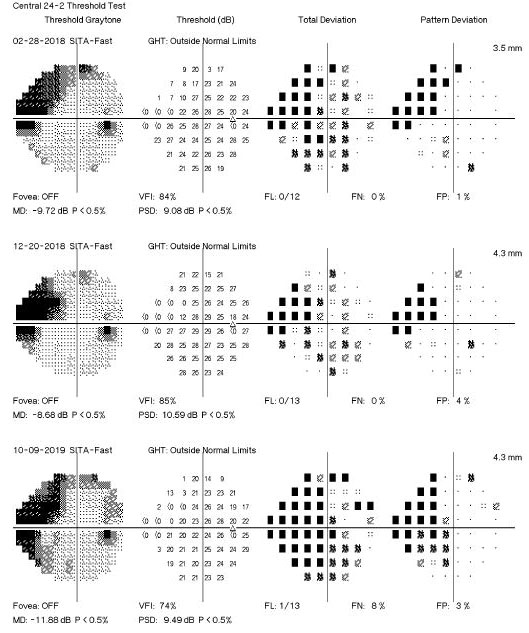 |
Fig. 3. This visual field readout shows event-based change, indicating visual field progression. Click to enlarge image. |
Despite these disadvantages, incisional surgery does provide an IOP reduction of 30% to 50% and should be strongly considered when the benefits of surgery outweigh the risks.18 Therapeutic surgical management should not only maintain the patients’ visual field and functional vision but also preserve their quality of life and independence.12,13 Clinicians must weigh the risks and benefits of incisional surgery and only recommend these options when absolutely critical to stabilize aggressive glaucomatous progression.
Table 3. Visual Field Progression26
|
Optometrists’ primary goal in the management of glaucoma is to ensure a lifetime of visual function to meet patients’ visual demands. No perfect formula exists to determine which therapeutic approach is best. By evaluating patients’ risk for visual decline, medication adherence and burden, and the pros and cons of surgery, clinicians can individualize a therapeutic plan to address any apparent progression—and preserve vision as long as possible.
Drs. Brian Fisher and David Johnson work at The Villages VA Outpatient Clinic, The Villages, Fla.
Dr. April Fisher is an optometrist at Ocala West VA Community Based Outpatient Clinic, Ocala, Fla.
1. Weinreb RN. Progression of Glaucoma: The 8th Consensus Report of the World Glaucoma Association. Amsterdam: Kugler Publications; 2011. 2. Weinreb RN. Diagnosis of Primary Open Angle Glaucoma: The 10th Consensus Report of the World Glaucoma Association. Amsterdam: Kugler Publications; 2017. 3. Cymbor M, Lifferth A. Progressing glaucoma: When to manage with meds, laser, and surgery. 2019 American Academy of Optometry Conference. Orlando, fL, October 24, 2019. 4. Sihota R, Angmo D, Ramaswamy D, Dada T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018;66:495-505. 5. Liebmann J, Weinreb RN. Medical Treatment of Glaucoma: The 7th Consensus Report of the World Glaucoma Association. Amsterdam: Kugler Publications; 2010. 6. Garg A, Vickerstaff V, Nathwani N, et al. Primary selective laser trabeculoplasty for open-angle glaucoma and ocular hypertension: Clinical outcomes, predictors of success, and safety from the laser in glaucoma and ocular hypertension trial. Ophthalmology. 2019;126(9):1238-48. 7. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-16. 8. Chi SC, Kang Y, Hwang D, Liu CJ. Selective laser trabeculoplasty versus medication for open-angle glaucoma: Systematic review and meta-analysis of randomised clinical trials. Br J Ophthalmol. February 12, 2020. [Epub ahead of print]. 9. Garg A, Gazzard G. Selective laser trabeculoplasty: Past, present, and future. Eye (London). 2018;32(5):863-76. 10. Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society. J Glaucoma. 2017;26(8):687-93. 11. Francis BA, Sarkisian SR, Tan JC. Minimally Invasive Glaucoma Surgery: A Practical Guide. New York: Thieme. 2017;1-2. 12. Janz NK, Wren PA, Lichter PR, et al. Quality of life in newly diagnosed glaucoma patients: the Collaborative Initial Glaucoma Treatment Study. Ophthalmol. 2001;108:887-97. 13. Pahlitzsch M, Klamann MKJ, Pahlitzsch M, et al. Is there a change in the quality of life comparing the micro-invasive glaucoma surgery (MIGS) and the filtration technique trabeculectomy in glaucoma patients? Graefes Arch Clin Exp Ophthalmol. 2017;255:351-57. 14. Lavia C, Dallorto L, Maule M, et al. Minimally invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS One. 2017;12(8):e0182142. 15. Weinreb RN. Glaucoma Surgery: The 11th Consensus Report of the World Glaucoma Association. Amsterdam: Kugler Publications; 2019. 16. Shah M. Micro-invasive glaucoma surgery - an interventional glaucoma revolution. Eye Vis (Lond). 2019;6:29. 17. Bhartiya S, Dhingra D, Shaarawy T. Revisiting results of conventional surgery: trabeculectomy, glaucoma drainage devices, and deep sclerectomy in the era of MIGS. J Curr Glaucoma Pract. 2019;13(2):45-49. 18. Zhou, M., Wang, W., et al. Trabeculectomy with verses without releasable sutures for glaucoma: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2014; 14(1):41. 19. MacDonald D. OCT interpretation for glaucoma diagnosis and management. 2019 American Academy of Optometry Conference. Orlando, fL, October 23, 2019. 20. Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119:1858-66. 21. Leung CK. Diagnosing glaucoma progression with optical coherence tomography. Curr Opin Ophthalmol. 2014;25:104-11. 22. Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96:47-52. 23. Mwanza JC, Durbin MK, Budenz DL, et al. Interocular symmetry in peripapillary retinal nerve fiber layer thickness measured with the Cirrus HD-OCT in healthy eyes. Am J Ophthalmol. 2011;151:514-21. 24. Sullivan-Mee M, Ruegg CC, Pensyl D, et al. Diagnostic precision of retinal nerve fiber layer and macular thickness asymmetry parameters for identifying early primary open-angle glaucoma. Am J Ophthalmol. 2013;156:567-77. 25. Mwanza JC, Oakley JD, Budenz DL, et al. Ability of Cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118:241-8. 26. Chu E, Hicks D. 50 Glaucoma facts: an evidence based overview for the primary care practitioner. 2019 American Academy of Optometry Conference. Orlando, fL, October 25, 2019. 27. Salazar D, Morales E, Rabiolo A, et al. Pointwise methods to measure long-term visual field progression in glaucoma. JAMA Ophthalmol. 2020;138(5):536-43. 28. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(6):S57-S68. 29. Schlote T, Schlote T, Kynigopoulos M, Kynigopoulos M. Selective laser trabeculoplasty (SLT): 1-year results in early and advanced open angle glaucoma. Int Ophthalmol. 2016;36(1):55-61. . |

