Trends and ControversiesCheck out the other feature articles in this month's issue: - Myopia: Should We Treat It Like a Disease? |
Most optometrists approach patient care from a clinical perspective, and rightly so. Direct examination of the eye is straightforward, painless and fruitful. However, breakthroughs in genetics and molecular biology have opened new avenues that begin at the most elemental level: our own DNA. The work of connecting these intrinsic factors to the reality of the patient in the chair is fraught with challenges—scientific, logistical, financial—but holds enormous potential, too. In fact, the first FDA-approved gene therapy was for a retinal degenerative disease. This has sparked significant interest in the role gene therapy plays in eye care, and where it’s headed in the future.
The nascent field of clinical genetics “will offer optometrists more avenues for diagnosis and treatment,” speculates Albert Morier, OD, MA, an associate clinical professor of ophthalmology at Albany Medical College. “As our understanding of ocular conditions and their molecular biology grows, we will be able to treat people more effectively. It will help us identify at-risk patients and determine the monitoring and treatment they need based on their genetic pattern.”
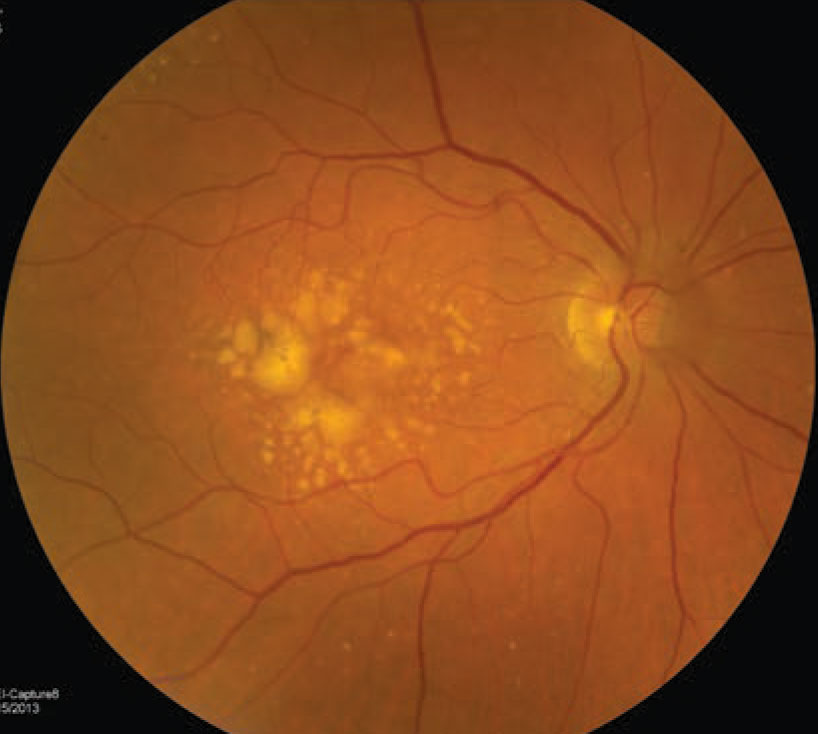 |
| The right eye of a 72-year-old white woman who has intermediate AMD with evidence of large drusen, drusenoid pigment epithelial detachment and hyperpigmentary changes. Based on the patient’s fundus photographs, we know the risk of developing late AMD, either neovascular and geographic atrophy, is as high as 50% in five years. Image courtesy of NEI. Click image to enlarge. |
While such enthusiasm is warranted, optometrists must also consider the practical questions and ethical implications raised by these genetic discoveries. Some advance our understanding but are years away from clinical value or raise the spectre of patients living in fear of an “inevitable” disease that may never manifest. But there are a few clear success stories that make the effort worthwhile.
What follows is a look at how genetics is informing, and in some cases advancing, the practice of eye care in four key spheres.
Retina
Genetics are at play in a number of retinal diseases, with both glowing successes and ongoing controversies:
Age-related macular degeneration (AMD). This complex disease is associated with multiple environmental and genetic risk factors. As researchers identify more genetic variants linked to AMD, the interest in developing genetic testing continues to grow.
Researchers have already developed gene-based AMD risk prediction models that account for disease status, genetic risk and lifestyle factors.1 In addition, two genetic tests for AMD risk assessment are now commercially available, ArcticDx and a recently launched option from Visible Genomics, a Chicago-based genetic testing company.2,3
However, the value of these genetic assessments remains controversial. Some argue that genetic tests overstate the risk of AMD and can lead to avoidable anxiety and unnecessary treatment.
The American Academy of Ophthalmology recommends clinicians avoid routine genetic testing for complex disorders such as AMD, at least until prospective clinical trials show specific benefits regarding surveillance or treatment.4
“While it can be beneficial to identify high-risk patients to ensure they are monitored appropriately, what are the medical ethics of telling someone at an early age that they have this gene?” explains Dr. Morier. “We have to consider these implications as well.”
The key is knowing how to use the information genetic testing provides, according to Emily Chew, MD, of the National Eye Institute/National Institutes of Health. “Without discernment, patients can be given a sentence that may not be appropriate,” Dr. Chew explains. “For some patients, genetic findings can cause anxiety needlessly. For others, it may provide a false sense of security. It is really important that patients understand that it’s just a number. It’s a finding that can be difficult to interpret, and routine eye exams remain vital.”
In addition, the debate rages on about whether genotyping should be standard care for AMD patients taking antioxidants and zinc. Research suggests that zinc in the Age-Related Eye Disease Studies (AREDS) formula actually increases progression risk in some individuals with specific genetic variants in CFH and ARMS2/HTRA. Three statistical teams from separate academic centers examined the data from AREDS, as well as the findings that support genetic testing, and determined that the data does not currently support genotyping for AMD and additional research is required.5 According to Dr. Morier, a community-based study shows that, among patients with neovascular AMD, those in the previously identified genetic zinc-risk group were three times more likely to have taken AREDS supplements than those in other genetic groups.6
“Genetic testing is important for research, but not for management at this point,” says Dr. Chew, who ran both the AREDS1 and AREDS2 studies. “I would love to be able to use genetic testing to personalize treatment, but we just don’t have the treatment or the genetic data to suggest that the patients respond differently because of genetic interactions.”
Retinitis pigmentosa (RP). Patients with this condition have very few therapeutic options due to, in part, the genetic complexities of the condition, which is linked to about 70 known genes and 3,000 genetic mutations.7 Additionally, other retinal degenerative diseases, including Leber’s congenital amaurosis, are genetically associated with RP. Gene therapy could offer new hope to this patient population.
One of the recent genetic breakthroughs in retinal diseases was the 2017 FDA approval of Luxturna (voretigene neparvovec, Spark Therapeutics), the first directly administered gene therapy approved in the United States. It is approved for the treatment of patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy.8
While an important step forward in genetics and eye care, voretigene is a limited therapy. It is only beneficial for the 1,000 to 2,000 patients in the United States with the recessive RPE65 mutation.8 Far more patients with other hereditary retinal degenerative diseases are still waiting for a viable gene therapy.
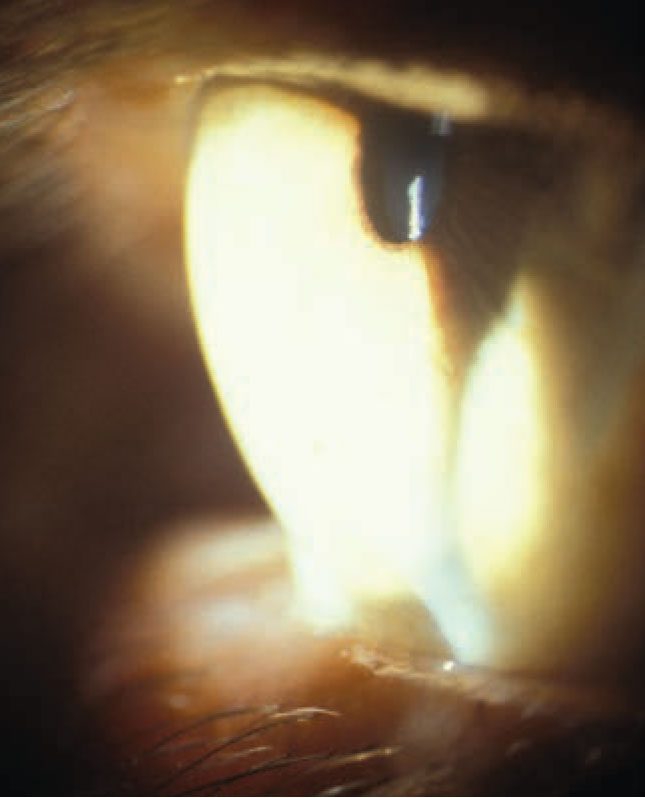 |
| Cornea steepening in a patient with keratoconus. Image courtesy of Joseph Shovlin, OD. Click image to enlarge. |
Additionally, this particular therapy calls attention to an ongoing challenge associated with personalized medicine: cost. At $425,000 per eye, it comes with a hefty price tag.7 Even though it’s a significant cost up-front, one study found voretigene neparvovec was cost effective over a lifetime, associated with lower total costs ($2.2 million vs. $2.8 million) and higher quality-adjusted life-year (18.1 vs. 8.6) compared with standard of care.9
Other conditions. Choroideremia (CHM), an x-linked recessive chorioretinal dystrophy that affects approximately one in 50,000 to100,000 individuals, currently has no approved treatment options. More than 100 variations in the CHM gene have been discovered, paving the way for potential gene therapies.10,11 A number of studies exploring gene therapies in these patients are underway, including the first Phase III trial for the treatment of choroideremia.12
The development of gene therapies for Stargardt’s disease, another rare retinal condition with a genetic inheritance pattern, has proven challenging because adeno-associated viruses (AAVs), the engineered pathogens used for gene therapies, are ineffective with the ABCA4 gene. To overcome this, a gene therapy in development uses dual-vector AAV technology. Researchers are also exploring another delivery method that uses chemically modified lipids.13 In animal models, they found that Stargardt’s did not return for up to eight months after treatment.13
“If you suspect your patient may have one of these conditions, genetic testing can confirm your suspicions, but it is important you make it clear that there is no treatment available at this time,” says Mohammad Rafieetary, OD, a consultative optometrist at a large retina practice in Germantown, TN. “In the future, however, having this genetic information could help them gain access to clinical trials and new therapies.”
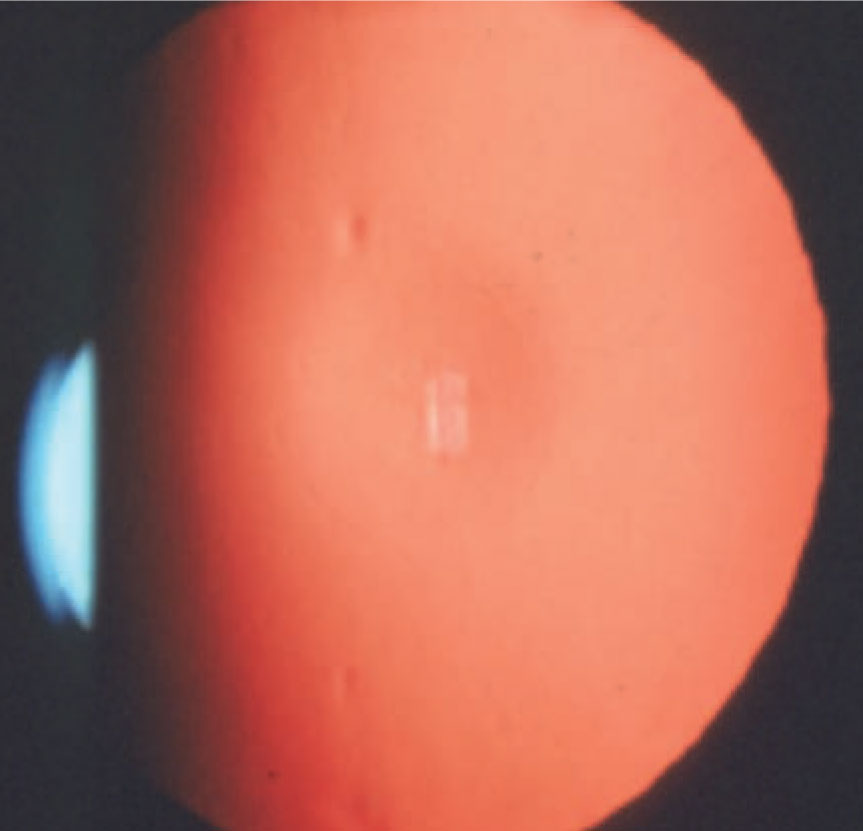 |
| Distorted retinoscopic reflex in a patient with keratoconus. Image courtesy of Joseph Shovlin, OD. Click image to enlarge. |
Cornea
Degenerations and dystrophies of the cornea require diligent screening, particularly to identify surgery contraindications such as keratoconus and transforming growth factor beta–induced (TGFB-I) dystrophies. Advancements in genetic screening can help clinicians recognize at-risk patients before they undergo refractive surgery, possibly saving patients from progression and exacerbation of their condition.
A growing understanding of the genetics behind keratoconus can help clinicians identify these patients before the condition becomes vision threatening. One study identified five genetic loci associated with corneal hysteresis and corneal resistance factor, which are linked to keratoconus.14 Another trial showed that the heritability of posterior corneal curvature was slightly higher compared with anterior corneal curvature.15 When examining corneal topographic measures, researchers reported that the index of surface variance, central keratoconus index and index of vertical asymmetry had the highest levels of heritability.
Testing for some corneal conditions is now possible with the AvaGen (Avellino) that examines more than 1,000 variants across 75 genes for keratoconus and over 70 mutations of the TGFBI gene for corneal dystrophies.16
“Identifying genetic predisposition can help monitor or even avoid disease progression, and that is key,” notes Joseph Shovlin, OD, of Scranton, PA. “For example, in keratoconus, you can make a diagnosis earlier and educate the patient as to what their options are, including crosslinking, which could—if used early enough—slow the progression of the disease.”
Unlike other conditions that don’t have viable treatment options, Dr. Shovlin recommends genetic testing for corneal diseases.
Fabry’s disease. There are a number of ocular manifestations of this condition, including cornea verticillata, distinctive lenticular opacities and vascular tortuosity of the conjunctiva and retina.17 Although identifying this disease can be challenging because several systemic drugs can cause the same presentation, early detection is key to reduce morbidity and mortality. Gene therapy is currently under investigation for this patient population.
MARVEL1, the first trial of an AAV-based gene therapy for Fabry’s disease, is currently studying the safety of FLT190. It is also looking at whether this treatment approach leads to continuous production of high alpha-GAL A levels.18 Interim data from another study supports the potential firstline use of the gene therapy AVR-RD-01.
The first patient treated with this therapy continued to show increased leukocyte and plasma AGA enzyme activity 22 months following treatment.19 While the other three patients in the study have a shorter follow-up, they are showing increased enzyme activity as well.
Other conditions. Fuchs’ corneal dystrophy, strongly associated with TCF4, is also of interest to geneticists.20 Researchers performed a genome-wide association study (GWAS) on 1,404 Fuchs’ cases and 2,564 controls.21 This was followed by a replication and meta-analysis, for a total of 2,075 cases and 3,342 controls. They identified three novel loci meeting genome-wide significance. Additionally, the researchers reported an overwhelming effect of the established TCF4 locus.
Researchers also linked the susceptibility and severity of microbial keratitis in contact lens wearers to genetic variants in different cytokine genes and DEFB1.22 Genetic susceptibility testing could one day play a role in addressing this condition and help ODs take preventive measures.
By using genome sequencing, researchers discovered the root cause of posterior polymorphous corneal dystrophy (PPCD), a rare autosomal-dominant corneal dystrophy. The investigators uncovered the variation to the DNA, which is located on GRHL2 gene, that causes dysfunction in the endothelial barrier and PPCD.23 This lays the groundwork for future study and potential therapies.
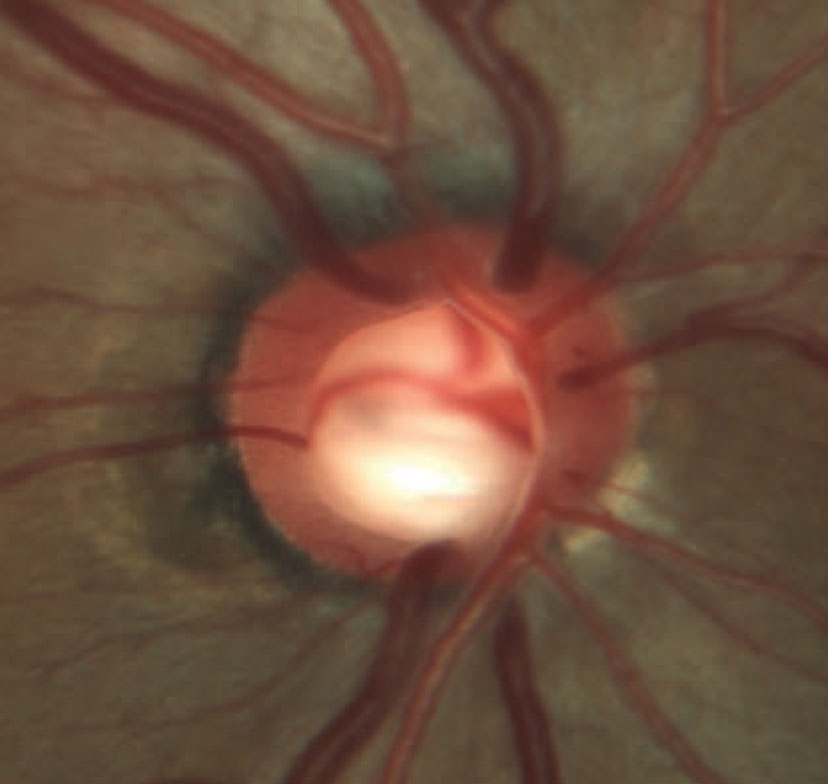 |
| Rim erosion and laminar remodeling in a patient with POAG. The total number of identified POAG loci has increased to 74 in the past few years, enhancing our understanding of the genetic aspects of this condition. Image courtesy of Andrew Rixon, OD. Click image to enlarge. |
Glaucoma
This condition can be divided into two groups: early-onset forms—e.g., juvenile open-angle glaucoma (JOAG), congenital glaucoma, anterior segment development syndromes—and adult-onset types such as primary open-angle glaucoma (POAG), angle-closure glaucoma and exfoliative glaucoma.24
Early-onset glaucomas, while rare, can have large biological effects and often involve multiple generations. Mutations in MYOC, the first gene to be implicated in glaucoma, are linked to familial JOAG.24 These patients often develop a severe form of glaucoma with a high intraocular pressure (IOP) that’s difficult to manage with current therapies.25 While intervention is key, many patients are asymptomatic and may not seek treatment until their disease has progressed. Therefore, identifying genes associated with JOAG and other early-onset glaucomas opens the door to genetic testing, which allows for early detection.
Adult-onset glaucoma can have a complex inheritance and is often associated with multiple genetic or environmental risk factors.24 Unlike early-onset disease, researchers do not have the same access to multiple generations, which makes it more challenging to explore genetic variants in this patient population. As a result, GWAS has become an important avenue to discovery. In 2017, 16 genomic regions were associated with POAG at a genome-wide level of significance. With rapid breakthroughs, the total number of identified POAG loci has increased to 74.26
The NEIGHBORHOOD Consortium—supported by the National Eye Institute—was founded in 2012 to gain a better understanding of the genomic architecture of glaucoma. Since then, researchers have collected data on more than 5,000 POAG cases and have conducted a number of genetic analyses. A meta-analysis of GWAS findings identified FOXC1, ATXN2 and TXNRD2 as susceptibility loci for POAG and potential therapeutic targets.27
Recent studies have proven the heritability of primary angle-closure glaucoma, and analyses confirm the presence of eight loci significantly associated with a risk of the disease.28 Current literature suggests a significant portion of normal-tension glaucoma (NTG) patients have a family history of glaucoma.29 There are a number of candidate genes for NTG, but further study is needed.29
Despite the growing body of literature, the immediate clinical implications remain unclear. Currently, genetic testing is only recommended when it will impact treatment or surveillance.30 For glaucoma, this typically applies to early-onset disease, such as JOAG, where patients could derive significant benefit. The applicability of widespread testing for other forms of glaucoma has not yet been established.
“While significant progress has been made, we’re still in the discovery phase,” notes Andrew Rixon, OD, an attending optometrist at the Memphis VA. “As we gain a better understanding of glaucoma and are able to predict these conditions earlier, we will be able to intervene sooner, offering patients a better quality of life with, hopefully, less disease burden and better outcomes.”
Until then, Dr. Rixon urges practicing clinicians to stay current on the latest research and discoveries. “As healthcare providers, we need to be aware of the research and its potential impact on our field and practice,” he says. “There’s enough information out there that our patients are going to come to us with questions and we have to be prepared to have those discussions.”
Vision Disorders
The genetic study of vision disorders such as myopia and achromatopsia has become a priority for many researchers in recent years.
Myopia. Researchers have uncovered up to 50 loci and genes by early linkage and candidate gene studies; however, these findings remain largely unverified by replication studies.31 A recent study of 3,300 children concluded that the ZC3H11B and BICC1 genes are risk factors for moderate and high myopia, and five genes—ZC3H11B, KCNQ5, SNTB1 and GJD2—increase a child’s risk of excessive axial length.32
The Consortium for Refractive Error and Myopia examined the genes of more than 250,000 individuals and found 139 independent susceptibility loci by single variant analysis and 22 additional loci through post-GWAS.33 While these findings provide a new understanding of myopia and its mechanisms, this analysis documents just 8% of the phenotypic variance, highlighting the need for additional exploration.31
Gene Therapies in the PipelineAs discoveries continue, more gene therapies will become a reality. Luxturna (voretigene neparvovec, Spark Therapeutics) was granted FDA approval in 2017 for patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy.8 Here is a rundown of where some other promising treatments stand: AMD
Stargardt’s Disease
Achromatopsia
Fabry’s Disease
|
“While hundreds of variants have been identified, it only explains a small percentage of the variability in refractive error,” notes Donald O. Mutti, OD, PhD, a professor at the Ohio State University College of Optometry. “Our understanding of the disease is growing, but these discoveries don’t describe as much of the trait as we would like. And so, today, the best way to predict myopia is to measure a child’s refractive error regularly.”
Even environmental factors associated with myopia, such as an individual’s level of education, are entangled with genetics. Myopia prevalence doubles among individuals who pursue higher education compared with those who do not, and research shows individuals with a high genetic load as well as university-level education had a significantly greater risk of myopia compared with those who only had one of these two factors.31,34 A GWAS for educational attainment identified 74 genome-wide significant loci associated with number of years of schooling completed.35
“The big question is, how much of myopia is genetic and how much is environmental?” notes Dr. Mutti. “This has prompted interest in the genes related to educational attainment and their connection to myopia. Is it about doing more near work, spending less time outdoors or inheriting some cognitive skill that makes for better success in school? Or is it some combination or interaction between heredity and environment?”
“Untangling this association between genes for educational attainment and myopia is where, in my opinion, genetics is heading,” Dr. Mutti adds.
According to Dr. Mutti, any possible genes related to educational attainment may help researchers better identify at-risk children who would particularly benefit from more time outdoors.
Achromatopsia. Six gene variants are associated with this condition, explaining greater than 90% of cases.36,37 The most prevalent are CNGA3 and CNGB3.37
While no treatments currently exist for achromatopsia, a number of gene therapy trials are underway, including one for AAV-CNGA3. The therapy, designed to restore cone function, is delivered to the cone receptors at the back of the eye via subretinal injection. In 2018, AAV-CNGA3 was granted orphan drug designation by the FDA.38
Recent findings from an open-label, nonrandomized controlled trial suggest that another gene therapy, AAV8.CNGA3, improved visual outcomes in nine patients with CNGA3-linked achromatopsia. The study found no significant safety issues.39 The study authors noted that future studies must explore this approach at an earlier age to determine if this will “lead to greater functional benefit because of higher cortical plasticity.”
A Look to the Future
While some ocular conditions are already witnessing the impact of genetic advancement, the exact timeframe for broad applications in optometric practice remains to be seen. As breakthroughs continue, it is crucial for optometrists to educate themselves on the latest research and engage in meaningful discussions around the potential impact—both positive and negative—these advances can have on their patients.
“Genetics-guided therapy is in our future and, as a field, we have to embrace this technology,” notes Dr. Rafieetary. “We have to stay on top of these subjects so, as they become more accessible and commonplace, we didn’t miss the boat. To optometrists I would say, stay tuned, study as much as you can and use common sense, evidenced-based medicine and standard of care when using any therapies or technologies.”
1. Morier A, Lewis R. Genetics in eye care. Rev Optom. 2013:150(6):68-75. 2. Visible Genomics launches DNA age-related macular degeneration testing. Vision Monday. September 17, 2020. 3. ArcticDX. Macula Risk PGx DNA test. arcticdx.com. Accessed September 17, 2020. 4. Warwick A, Lotery A. Genetics and genetic testing for age-related macular degeneration. Eye. 2018;32:849-57. 5. Assel M, Li F, Wang Y, et al. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2018;125(3):391-7. 6. Kaufman SR, Yoganathan P, Small KW, et al. Genetics and Age-Related Eye Disease Study formulation interaction in neovascular age-related macular degeneration. J VitreoRetinal Dis. August 19, 2020. [Epub ahead of print]. 7. Barnett J, Landa G. A new gene therapy for early-onset RP. Rev Ophthalmol. 2018:25(11):57-61. 8. U.S. Food & Drug Adminstration. FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. 2017. www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss. Accessed August 16, 2020. 9. Johnson S, Buessing M, O’Connell T, et al. Cost-effectiveness of voretigene neparvovec-rzyl vs standard care for RPE65-mediated inherited retinal disease. JAMA Ophthalmol. 2019;137(10):1115-23. 10. Chan S, Bubela T, Dimopoulos I, et al. Choroideremia research: report and perspectives on the second international scientific symposium for choroideremia. Ophthalmic Genet. 2016;37(3):267-75. 11. Freund P, Sergeev Y, MacDonald I. Analysis of a large choroideremia dataset does not suggest a preference for inclusion of certain genotypes in future trials of gene therapy. Mol Genet Genomic Med. 2016;4(3):344-58. 12. Choroideremia Research Foundation. Nightstar announces first ever phase 3 choroideremia gene therapy trial. www.curechm.org/2017/10/nightstar-announces-first-ever-phase-3-choroideremia-gene-therapy-trial. Accessed August 16, 2020. 13. Sun D, Schur R, Sears A, et al. Non-viral gene therapy for Stargardt disease with ECO/pRHO-ABCA4 self-assembled nanoparticles. Mol Ther. 2020;28(1):293-303. 14. Khawaja A, Rojas Lopez k, Hardcastle A, et al. Genetic variants associated with corneal biomechanical properties and potentially conferring susceptibility to keratoconus in a genome-wide association study. JAMA Ophthalmol. 2019;137(9):1005-12. 15. Heydarian S, Hashemi h, Yekta A, et al. Heritability of corneal curvature and pentacam topometric indices: a population-based study. Eye Contact lens. 2019;45(6):365-71. 16. Avellino. AvaGen. www.avellino.com/en/products/avagen-test. Accessed September 2, 2020. 17. Samiy N. Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder. Surv Ophthalmol. 2008;53(4):416-23. 18. Lopes JM. First patient dosed in phase 1/2 trial of Fabry disease gene therapy candidate FLT190. Fabry Disease News. 2019 19. AVROBIO Reports Updated Clinical Data from Investigational Gene Therapy Programs for Fabry Disease and Cystinosis. www.businesswire.com/news/home/20200513005165/en/AVROBIO-Reports-Updated-Clinical-Data-Investigational-Gene. May 13, 2020. Accessed September 1, 2020. 20. Baratz K, Tosakulwong N, Ryu E, et al. E2-2 protein and Fuchs’s corneal dystrophy. N Engl J Med. 2010;363(11):1016-24. 21. Afshari N, Igo R, Morris N. et al. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nat Commun. 2017;8:14898. 22. Genetics of Rare Corneal Dystrophy Identified. Review of Optometry. March 28, 2018. www.reviewofoptometry.com/article/genetics-of-rare-corneal-dystrophy-identified. Accessed August 16, 2020. 23. Liskova P, Dudakova L, Evans C, et al. Ectopic GRHL2 expression due to non-coding mutations promotes cell state transition and causes posterior polymorphous corneal dystrophy 4. Am J Hum Genet. 2018;102(3):447-59. 24. Wiggs J. The genetic component: Is a curative or preventive therapy on the horizon? Glaucoma Today. 2019 November/December:43-9. 25. Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet. 2017;26(R1):R21-R27. 26. Choquet H, Wiggs JL, Khawaja AP. Clinical implications of recent advances in primary open-angle glaucoma genetics. Eye (Lond). 2020;34(1):29-39. 27. Cooke BJ, Loomis S, Kang J, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open angle glaucoma. Nat Genet. 2016;48(2):189-94. 28. Wang J, Yusufu M, Chuen Khor C, et al. The genetics of angle closure glaucoma. Experimental Eye Research. 2019;189:107835. 29. Trivli A, Koliarakis I, Terzidou C, et al. Normal-tension glaucoma: Pathogenesis and genetics. Exp Ther Med. 2019;17(1):563-74. 30. Wade M. Glaucoma genetics: when is testing warranted? EyeNet. June 2016. www.aao.org/eyenet/article/glaucoma-genetics-when-is-testing-warranted. Accessed August 16, 2020. 31. Tedja M, Haarman A, Meester-Smoor M, et al. IMI–myopia genetics report. Invest Ophthalmol Vis Sci. 2019;60(3):M89-M105. 32. Li FF, Lu SY, Tang SM, et al. Genetic associations of myopia severities and endophenotypes in children. Br J Ophthalmol. August 14, 2020. [Epub ahead of print]. 33. Tedja MS, Wojciechowski R, Hysi PG, et al. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet. 2018;50(6):834-48. 34. Verhoeven VJ, Buitendijk GH, Consortium for Refractive Error and Myopia (CREAM), et al. Education influences the role of genetics in myopia. Eur J Epidemiol. 2013;28(12):973-80. 35. Okbay A, Beauchamp JP, Fontana MA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539-42. 36. Hirji N, Aboshiha J, Georgiou M, et al. Achromatopsia: clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018;39(2):149-57. 37. Kohl S, Varsanyi B, Antunes GA, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13(3):302-8. 38. MeriaGTx. Meiragtx announces AAV-CNGA3 granted rare pediatric disease designation by the U.S. FDA for the treatment of achromatopsia. https://meiragtx.gcs-web.com/node/6681. August 28, 2018. Accessed August 16, 2020. 39. Fischer MD, Michalakis S, Wilhelm B, et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia: a nonrandomized controlled trial. JAMA Ophthalmol. 2020;138(6):643-51. 40. Sabbagh O, Mehra A, Maldonado R. Gene therapy in AMD: Promises and challenges. Retina Specialist. 2020:6(2):16-19. 41. ADVM-022 intravitreal gene therapy for wet AMD (OPTIC). https://clinicaltrials.gov/ct2/show/NCT03748784. Accessed August 16, 2020. 42. Fight Blindness Foundation. AGTC announces development of Stargardt disease gene therapy. www.fightingblindness.org/research/agtc-announces-development-of-stargardt-disease-gene-therapy-41. November 7, 2019. Accessed August 16, 2020. 43. Dyka FM, Molday LL, Chiodo VA, et al. Dual ABCA4-AAV vector treatment reduces pathogenic retinal A2E accumulation in a mouse model of autosomal recessive Stargardt disease. Hum Gene Ther. 2019;30(11):1361-70. |

