Fortunately, recent years have witnessed considerable progress in our understanding of the pathophysiology of DME, which has in turn led to growth in the development of new therapies. The once-subdued field of DME treatment is suddenly flourishing.
The goal of this review is to briefly discuss our understanding of DME, as well as to elucidate the current state of ocular treatment options––from mainstays to the most recent clinical trials for this complex condition. As diabetes rates continue to rise, we will likely see more of these patients in our offices, so it is in our professional interest to stay informed about current developments.
DME 101
 |
|
|
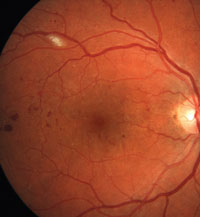
1. Diabetic retinopathy with suspected macular edema. Note the hard exudates located within 500µm of the fovea's center.
|
|
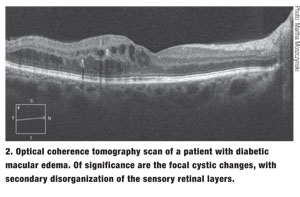 |
Diabetic macular edema is the most frequent cause of vision loss related to diabetes.2-4 The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) found the 14-year incidence of DME in patients with type 1 diabetes to be 26%.5 Similarly, the Diabetes Control and Complications Trial (DCCT) reported that nearly 27% of type 1 diabetes patients develop DME within nine years of onset.6 For type 2 insulin-dependent patients, the 10-year incidence is 25.4% and for type 2 non-insulin dependent individuals that figure is 13.9%. Because almost 26 million children and adults in the US have diabetes—a staggering 8.3% of the population—a large number of patients are at risk for DME.7
The pathogenesis of diabetic macular edema is multifactorial. It occurs primarily through a disruption of the blood/retinal barrier (BRB) and subsequent accumulation of fluid, inflammatory material and growth factors within the layers of the macula.2,8 The breakdown of retinal integrity and leakage of vasoactive factors may lead to the storage of pro-inflammatory factors in the vitreous.8
Laser Photocoagulation
For the first 25 years following the publication of the ETDRS trial in 1985, thermal laser was the mainstay treatment for DME. Specifically, the indication was for “prompt, focal/grid laser to microaneurysms or areas of macular edema.”1 Success following such laser treatments was defined as reducing by half the number of patients who experienced a doubling of the visual angle (i.e., going from 20/40 to 20/80 visual acuity).
Although panretinal photocoagulation, as performed during the ETDRS trial, was successful in reducing rates of visual loss due to DME, there were a few problems. Many patients did not recover lost vision, and a subset of patients were unresponsive to therapy.9 In addition, a subset of patients exhibited diabetic pathology near the fovea, where laser would cause permanent structural damage and potentially dense central scotomas.
Anti-VEGF Therapy
The need for a treatment for patients whose macular edema involved the fovea ignited the search for non-structurally damaging intraocular pharmacologic agents. The Diabetic Retinopathy Clinical Research Network (DRCR.net) compared standard laser therapy with combination therapy (laser plus intravitreal anti-VEGF), and laser therapy against intravitreal anti-VEGF alone. The researchers found superior visual outcomes among patients who received combined injection and focal laser vs. those who received focal laser alone. Further, they suspect anti-VEGF agents decrease the release of prostaglandins (thus curbing inflammation) and inhibit expression of the VEGF gene, thereby decreasing neovascularization.9
• Lucentis. In August 2012, ranibizumab (Lucentis, Genentech)––previously FDA-approved for intravitreal injection in patients with AMD––gained FDA approval to treat DME. Two subsequent trials, RESTORE and REVEAL, demonstrated the usefulness of ranibizumab.4
The RESTORE trial indicated that ranibizumab as monotherapy or in combination with macular laser provided superior visual acuity over patients treated with only macular laser.
The REVEAL trial was similar to the RESTORE, except that it followed an Asian cohort. It also validated the superior outcome of ranibizumab and ranibizumab plus laser when compared to laser monotherapy.
• Avastin. Another anti-VEGF therapy, bevacizumab (Avastin, Genentech)––approved for intravenous use in cancer patients––has been used off-label as an intravitreal injection to treat both AMD and macular edema.10 Bevacizumab performed well in the RIDE, RISE and RESTORE trials.10,11
Additionally, in the Bevacizumab or Laser Therapy (BOLT) study, patients who received bevacizumab injections every six weeks exhibited superior visual acuity compared to those who received macular laser therapy every four months.12
• Eylea. The newest addition to the ocular anti-VEGF injection family is aflibercept (Eylea, Regeneron). The agent features the same mechanism of action as ranibizumab and bevacizumab, but has a higher binding affinity for VEGF that can maintain therapeutic effect for up to three months.9 In some patients, the injection provides extended action, meaning that less frequent dosing may be possible compared to other anti-VEGF agents.
The DME and VEGF Trap-Eye: Investigation of Clinical Impact (DAVINCI) trial demonstrated that at six months post-injection aflibercept produced better visual outcomes than laser photogoagulation.
Currently, the VISTA (VEGF Trap-Eye In Vision Impairment Due to DME) study is ongoing. It is a three-year trial comparing aflibercept to laser monotherapy, and the manufacturer hopes the FDA will approve it for DME.11
Among anti-VEGF injectables, side effects are rare but include retinal detachment and endophthalmitis. More unlikely side effects include raised intraocular pressure and accelerated cataract formation.3
Corticosteroids
Another category of ocular injectables is the corticosteroids. There is mounting evidence that inflammation may play a significant role in the development of DME.2,9 It is suspected that activation of inflammatory mediators in the vitreous causes subsequent adhesion and endothelial cell damage.2 Endothelial cell death then contributes to the breakdown of the BRB and can lead to fluid accumulation in the macula.2,9 The use of steroids is thought to curb the inflammatory breakdown of the BRB, inhibit the expression of VEGF and decrease the release of prostaglandins.2,8,9,13
Most injectable corticosteroids are administered intravitreously. Triamcinolone acetonide (Kenalog, Bristol-Myers Squibb) is the most widely used, and was studied as the primary treatment for DME by the DRCR.net researchers. Results showed that while triamcinolone acetonide may be less effective than laser photocoagulation as a primary treatment, it may benefit eyes that are otherwise unresponsive to laser treatment.8 Side effects of intravitreal corticosteroids include accelerated development of cataracts and elevated intraocular pressure––the latter of which may lead to glaucomatous optic nerve damage.3
A newer formulation of intravitreal corticosteroid, a preservative-free version of triamcinolone called Triesence (Alcon), was approved by the FDA to treat sympathetic ophthalmia, temporal arteritis, uveitis and ocular inflammatory conditions unresponsive to topical corticosteroids.13 Triesence could be used off-label to treat DME. Its advantage over Kenalog lies in the preservative issue: triamcinolone acetonide contains benzyl alcohol, which could be toxic to the eye. Triesence obviates such concerns.
Intravitreal corticosteroids also may be delivered via sustained-release implant. The two primary technologies—fluocinolone (Retisert, Bausch + Lomb) and dexamethasone (Ozurdex, Allergan)—reduce injection frequency, but are relatively expensive. This approach may be too aggressive for many DME patients, however. The application of Retisert requires intraocular surgery, including a 4mm sclerotomy. Potential adverse effects of such a surgery include endophthalmitis, accelerated cataract formation, retinal detachment, vitreous hemorrhage and hypotony.
It is worth noting that these agents are FDA approved for different indications. Retisert, purported to last approximately 30 months, is approved for chronic non-infectious uveitis. Ozurdex, whose sustained release is thought to last one to three months, is approved for macular edema following branch retinal vein occlusion or central retinal vein occlusion, and for the treatment of non-infectious uveitis in the posterior segment.
The potential injection-related adverse effects of these corticosteroid implants are similar to those of the anti-VEGF agents. However, steroids appear to increase risks for both elevated intraocular pressure and formation of cataracts. Short-term, the side effects are well tolerated, but a three-year follow-up of dexamethasone implants demonstrated high rates of cataract and glaucoma requiring surgical intervention.8
Finally, some intraocular steroids can be delivered as a sub-Tenon’s injection (e.g., triamcinolone). In this instance, because the drug enters the ocular environment through a periocular route, the side-effect profile is much safer. However, patients are at risk for infection, discomfort and subconjunctival hemorrhage.
Vitrectomy
Although it does not receive the level of attention that new therapies like anti-VEGF and steroids garner, surgical intervention still has a place in the treatment algorithm. There are a number of arguments in favor of vitrectomy when photocoagulation and intravitreal injections prove ineffective.
The role of the vitreous in DME is thought to be twofold: (1) mechanical traction on the vitreoretinal interface, and (2) an accumulation of factors altering vascular permeability (e.g., VEGF) in the vitreous.3,8,9 One study indicated that vitreomacular separation was associated with an increased rate of spontaneous resolution of macular edema.9
Pars plana vitrectomy with membrane peel is the primary surgical option. This is an outpatient procedure in which the vitreous gel is removed from the eye, most often including an internal limiting membrane peel, thus removing both potential culprits. Sometimes macular surgery might include injection of a gas, either C3F8 or SF6, which may require patients to remain face-down for a few days, in some rare cases for more than a week. Though patients may experience varying levels of postoperative discomfort, success rates are fairly high.
In the DRCR.net research, vitrectomy for DME was studied in patients with moderate vision loss and vitreomacular traction. Postoperatively, 68% had a 50% reduction in macular thickness at six months.14 Visual acuity improved by 10 letters in 38% of patients and deteriorated by 10 letters in 22%. Preliminary studies by the DRCR network reported six-month and one-year outcome results after vitrectomy. At six months, both visual acuity and macular thickening improved in 28% to 49% of patients and worsened in 13% to 31%.
Postoperative complications after vitrectomy include retinal detachment, endophthalmitis, vitreous hemorrhage, elevated intraocular pressure and cataract progression.9
At two-year follow-up, the researchers determined that focal/grid laser produced visual acuity gains of two lines or more in one-third of eyes with center-involved DME. However, one-fifth of the laser-treated eyes lost two or more lines of visual acuity over the same time period—thus, the search for more effective therapies continues.9
Pharmacologic means can also achieve vitreolysis. Ocriplasmin (Jetrea, ThromboGenics) is a proteolytic enzyme that targets fibronectin and laminin, components of the vitreoretinal interface.16 In October 2012, the FDA approved Jetrea as an intravitreal injection for symptomatic vitreomacular adhesion (VMA). Approval was based on two clinical studies that collectively found VMA resolved in 26% of patients treated with Jetrea compared to 10% of those treated with placebo. Side effects were similar in both groups and included floaters, subconjunctival hemorrhage (from injection site), eye pain and blurred vision. At this time, no clinical trials of Jetrea in DME or diabetic retinopathy are underway. However, ThromboGenics is working with Bicycle Therapeutics to develop novel DME therapies that use bicyclic peptides to reduce vascular permeability.
Metabolic Considerations
Although we’ve chiefly focused on ocular therapeutic options for DME, it would be remiss not to mention another crucial topic regarding the diabetic patient: metabolic control. The DCCT study of patients with type 1 diabetes and the WESDR established that a higher level of glycosylated hemoglobin is a significant risk factor for DME.9 It has also been well established that tight control of hyperlipidemia and systemic hypertension lead to a lower rate of micro-vascular complications.9,16 Working with the patient to achieve optimal systemic medical control allows for the best prognosis following ophthalmic therapy.
The ocular effects of diabetes resound loudly in terms of vision impairment. In our fight against these afflictions, focal or grid laser photocoagulation remains the first-line treatment in a majority of patients with non–center-involving DME.3 For the latter scenario, intravitreal injection of anti-VEGF or corticosteroids is more commonly used.9 For those unresponsive to injections, vitrectomy may be a viable option. Early detection and treatment of diabetic macular edema is vital in preserving vision in the diabetes patient. As new and improved interventions are developed, treatment regimens will also change, and so will our conversations with our patients.
Dr. Fabrykowski is on staff at the Manhattan Eye, Ear and Throat Hospital Faculty Ophthalmology Practice, operated by Lenox Hill Hospital, in New York. She thanks William Schiff, MD, for assistance with this manuscript.
1. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985 Dec;103(12):1796-806.
2. Wenick AS, Bressler NM. Diabetic macular edema: current and emerging therapies. Middle East Afr J Ophthalmol. 2012 Jan;19(1):4-12.
3. Ranchod TM, Fine SL. Primary treatment of diabetic macular edema. Clin Interv Aging. 2009;4:101-7.
4. Shah R, Regillo C. The changing face of treatment for DME. Rev Ophthalmol. 2012 Dec;19(12):34-40.
5. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984 Dec;91(12):1464-74.
6. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control andComplications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology. 1995 Apr;102(4):647-61.
7. American Diabetes Association. Diabetes Statistics. Available at: www.diabetes.org/diabetes-basics/diabetes-statistics/. Accessed November 20, 2013.
8. Chan WC, Tsai SH, Wu AC, et al. Current treatments of diabetic macular edema. Int J Gerontology. 2011;5:183-8.
9. Kulkarni AD, Ip MS. Diabetic macular edema: therapeutic options. Diabetes Ther. 2012 Nov;3(1):1-14.
10. Haritoglou C, Neubauer A, Pringlinger S, et al. Retina 26.9 (Nov-Dec 2006): 999-1005. National Center for Biotechnology Information. US National Library of Medicine.
11. Bethke WC. DME: What Trials Tell Us About Treatment. Rev Opthalmol. 2012 Aug;19(8):30-4.
12. Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010 Jun;117(6):1078-86.e2.
13. Javey G, Schwartz SG. Emerging pharmacotherapies for diabetic macular edema. Exp Diabetes Res. 2012;2012:548732.
14. Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating Ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010 Jun;117(6):1064-77.e35.
15. Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012 Aug 16;367(7):606-15.
16. Gordon B, Chang S, Kavanagh M, et al. The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol. 1991 Oct 15;112(4):385-91.

