So, how exactly have these technologies changed the way we evaluate our patients? Is it time to get rid of our ophthalmoscopes? How has retinal imaging changed the way we look at the fundus? And, what techniques and technologies must we incorporate into our practices to appropriately manage our patients?
To answer these questions, we’ve enlisted the help of a few leaders in optometric retina care for their insights.
Dilation and Lenses
As our patients get older, their risk for eye diseases increases, which requires more frequent dilated exams. But what about younger, healthier patients? How often should they be dilated?
The American Optometric Association’s Clinical Practice Guidelines recommend a comprehensive exam in asymptomatic, risk-free patients every two years between the ages of six to 60, and then annual exams thereafter.1 While there are no specifics on frequency of dilation in the risk-free patient, the guidelines recommend using the initial dilated examination to guide the timing of subsequent dilations.
 Jeffry Gerson, OD, of WestGlen Eyecare & Omni Eye Centers of Kansas City, recommends a dilated retinal exam on all new patients. Then, assuming this baseline exam shows no pathology, Dr. Gerson generally dilates at intervals of every few years in young, healthy patients who have no family history of retinal disease.
Jeffry Gerson, OD, of WestGlen Eyecare & Omni Eye Centers of Kansas City, recommends a dilated retinal exam on all new patients. Then, assuming this baseline exam shows no pathology, Dr. Gerson generally dilates at intervals of every few years in young, healthy patients who have no family history of retinal disease.
For viewing the retina, newer generation non-contact slit lamp lenses are gaining increased popularity. In particular, the Digital Wide Field Lens (Volk Optical) seems to be a favorite among the doctors we interviewed. It has the ability to provide views past the equator, which is useful when performing a non-dilated exam. “It is a big step up from the traditional 90D and 78D lenses, offering better optics and less internal reflectivity,” says Steven Ferrucci, OD, chief of optometry at the VA Ambulatory Care Center in Sepulveda, Calif.
Another valuable lens is the Digital High Mag (Volk Optical), which allows for a more magnified and stereoscopic view of the macula and optic nerve. (See “Don’t Ditch the Direct Ophthalmoscope.”)
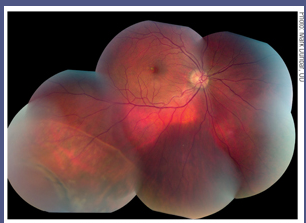 |
| Dilation and fundus photography are important elements of the retinal exam that, in many ways, cannot be replaced.
|
Fundus photography is useful for clinical photo-documentation of various retinal pathologies, such as optic nerve disorders, maculopathies, diabetic retinopathy and choroidal nevi.
“Although fundus photography doesn’t have quite the detail of newer imaging systems, it still provides important information on progression of retinal disease and is still a very important part of the retinal exam,” says Mark Dunbar, OD, director of optometric services at Bascom Palmer Eye Institute, in Miami.
Of note, fundus imaging is particularly useful for multiple-doctor practices that share follow-up of patient care because it decreases, at least to some extent, interpreter-related variability in disease monitoring, Dr. Ferrucci says.
Widefield retinal imaging, such as the Optomap (Optos), allows for nearly full diagnostic views of the fundus without the need for dilation. But the technology has become a topic of debate among eye doctors, some of whom question whether it replaces a dilated fundus exam, and if such imaging technology can be used to monitor retinal pathology.
Even the doctors we interviewed held different opinions on the use of widefield imaging. In the end, however, they formed a general consensus: Even with the improved magnification and contrast, wide-field imaging should not be used solely in the management of retinal disease, but as an adjunct to dilated retinal exams. “There’s still something about actually looking inside your patients’ eyes with a 90D or 78D lens that cannot be replaced,” Dr. Ferrucci says.
Optical Coherence Tomography
Optical coherence tomography (OCT) provides cross-sectional views of internal tissue previously only achievable by histological studies. Thus, OCT has been described as a method for non-invasive tissue ‘‘biopsy.’’2
Almost as fast as it takes to acquire a macular scan, the OCT has changed the way we look at the retina. With its detailed and instantaneous imaging of retinal structures, the OCT has quickly surpassed many of its technological antecedents for monitoring retinal disease. “We can now see types of disease and disease processes that we could really only surmise before. It’s really given us a whole new level of diagnosis,” Dr. Ferrucci says.
The earlier generation time-domain OCT (TD-OCT) uses a reference mirror that measures the time it takes for light to be reflected, acquiring approximately 400 scans per second. This results in longer acquisition times and lower resolution compared with the newer generation spectral-domain OCT (SD-OCT). The latter is able to acquire a significantly greater number of scans per second at a markedly higher resolution.
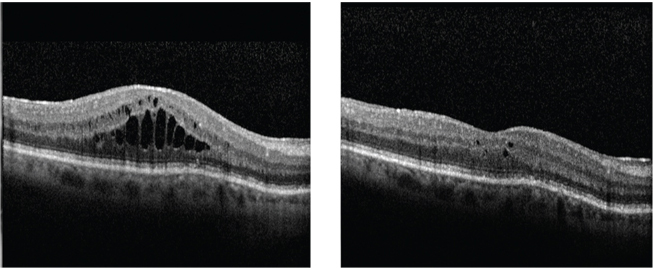 |
| OCT reveals macular edema in a patient one month after cataract surgery (left). One month after topical steroid and NSAID treatment (right), the edema shows significant improvement on OCT.
|
“From an optometric perspective, OCT has provided a way to look at the retina and correlate what you are seeing clinically to what is happening anatomically,” Dr. Dunbar says.
Specifically, SD-OCT has made it much easier to manage various retinal pathologies such as macular degeneration, epiretinal membranes and central serous chorioretinopathy.
In managing macular degeneration, OCT has the ability to objectively monitor the quantity and volume of drusen. Dr. Dunbar says the use of OCT for tracking macular degeneration is akin to the use of visual fields in following glaucoma. That is, “OCT allows us to quantify and document any changes or progression of drusen over time with serial imaging,” he says.
Speaking of glaucoma, OCT is also being used for optic nerve evaluation. Glaucoma analysis databases can follow progression of optic neuropathy due to glaucoma.
Another novel development: Vitreomacular traction was once a diagnosis optometrists and ophthalmologists could only surmise, but now it can easily be identified with OCT.3
OCT is also being used with fundus fluorescein angiography to follow wet macular degeneration and macular edema. Indeed, OCT has led retina specialists to rely much less on fluorescein angiography, Dr. Dunbar says. For instance, with the advent of OCT, the use of fluorescein angiography has declined by approximately 70% to 80% at Bascom Palmer Eye Institute, he estimates.
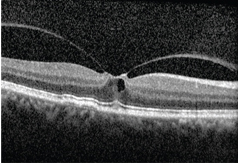 |
| OCT shows us images not easily visualized
before, such as this vitreomacular traction. |
It’s important, however, to not rely solely on the results of OCT without placing it in context. It should only be used when there is a clear indication. “The most important things are taking a careful history, listening to your patient and actually doing a good exam,” Dr. Ferrucci says. “So, as fantastic as OCTs are, we can’t throw everything else we’ve been doing out of the window and rely only on them. OCTs are another piece of the clinical puzzle. Granted, OCT is an important and impressive piece, but it shouldn’t be the only piece and it shouldn’t overrun our common sense.”
Fundus Autofluorescence
Fundus autofluorescence (FAF) is becoming a hot topic in the optometric world. More and more manufacturers are producing retinal cameras, fundus imagers and OCTs that include autofluorescence. It has become of particular interest in managing patients with macular degeneration. FAF allows for the visualization and distribution of lipofuscin, which may predict the progression of macular degeneration.5
Autofluorescence is not just for macular degeneration, Dr. Dunbar says. He also uses FAF in patients with central serous chorioretinopathy to show damage within the retinal pigment epithelium that wouldn’t be visible on fundus photography or OCT.
What should be included in the retinal diagnostic arsenal of a primary care optometrist? All of our experts agree that, at the very least, a primary care optometry practice should be equipped with a fundus camera.
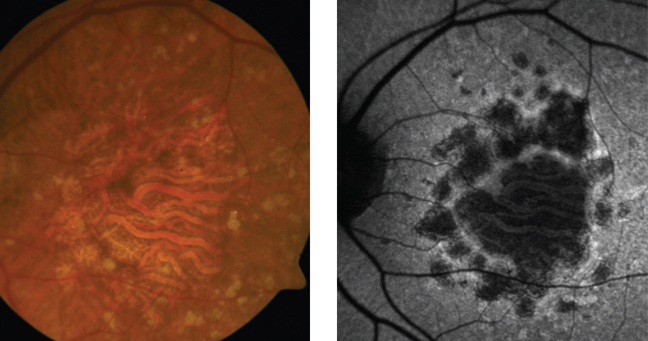 |
| Highlighted areas on fundus autofluorescence image show drusen,
while darkened areas show geographic atrophy. |
 Dr. Gerson also suggests that optometrists have an OCT “because it’s becoming standard of care in many diseases.” For instance, the multi-functionality of the OCT in detecting and following macular pathology, optic nerve disorders, hydroxychloroquine maculopathy and even neurological disease (such as multiple sclerosis) makes it an invaluable tool. “The OCT is becoming as common as the phoropter in the optometric office,” Dr. Dunbar says. Even a busy solo practice can justify purchasing a unit.
Dr. Gerson also suggests that optometrists have an OCT “because it’s becoming standard of care in many diseases.” For instance, the multi-functionality of the OCT in detecting and following macular pathology, optic nerve disorders, hydroxychloroquine maculopathy and even neurological disease (such as multiple sclerosis) makes it an invaluable tool. “The OCT is becoming as common as the phoropter in the optometric office,” Dr. Dunbar says. Even a busy solo practice can justify purchasing a unit.
“Not everyone can afford every instrument, and that’s OK,” Dr. Gerson says. “But it’s important for optometrists to be aware of what technologies are out there and why their patients will benefit from them. Not having a certain device doesn’t mean that your patient doesn’t deserve it. So, know where to send your patient to have that particular testing done. If another OD down the street has what your patient needs, you can always send the patient to them for that test.”
Dr. Trottini is in practice at Outlook Eyecare, in Monroe Township, NJ. Dr. Tolud is in practice at South Jersey Eye Physicians, in Cream Ridge, NJ.
1. American Optometric Association Consensus Panel on Comprehensive Adult Eye and Vision Examination. Optometric Clinical Practice Guideline: Comprehensive Adult Eye And Vision Examination, 2nd ed. St. Louis: American Optometric Association; 2005:10-11,15.
2. Costa RA, Skaf M, Melo LA Jr, et al. Retinal assessment using optical coherence tomography. Prog Retin Eye Res. 2006 May;25(3):325-53.
3. Gallemore RP, Jumper JM, McCuen BW 2nd, et al. Diagnosis of vitreoretinal adhesions in macular disease with optical coherence tomography. Retina. 2000;20(2):115-20.
4. Oberwahrenbrock T, Schippling S, Ringelstein M, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305.
5. Bearelly S, Khanifar AA, Lederer DE, et al. Use of fundus autofluorescence images to predict geographic atrophy progression. Retina. 2011 Jan;31(1):81-6.

