 |
Dry eye therapy has taken several unexpected turns over the years, with a new class of drugs and several new formulations of a tried-and-true therapy. Now, it seems neuroscience is taking a turn at the wheel. Scientists have discovered that the trigeminal nerve plays a critical role in ocular surface health and symptomatology. Although a misalignment of the eyes can fatigue the trigeminal nerve and cause dry eye symptoms, stimulating the trigeminal nerve can also promote tear production.
Research shows the parasympathetic nervous system (PNS), via the trigeminal parasympathetic pathway, controls tear film homeostasis through innervation of the lacrimal functional unit (LFU), which includes the cornea, conjunctiva and the structures that secrete tear film components, such as the lacrimal glands, meibomian glands and goblet cells.1-3 The PNS can most easily be accessed through the nose (Figure 1). In fact, 34% of basal tear production is due to sensory stimulation from inhaled air through the nasal passage.4
Investigators have developed or are developing nasal neurostimulation devices and pharmaceuticals to take advantage of this nasal LFU access point—a novel approach to producing a complete tear film in patients with dry eye disease.
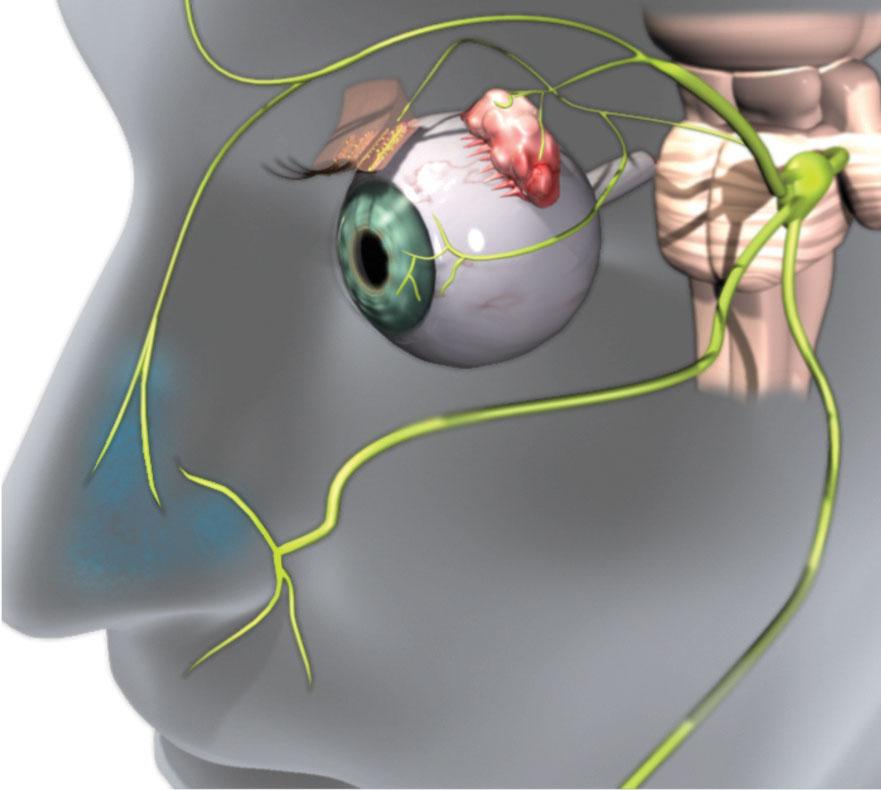 |
| Fig. 1. Efferent parasympathetic nerves innervate the LFU. The 0.1% dose of the OC-01 nAChR agonist nasal spray that targets these nerves led to a statistically significant improvement in dry eye symptoms. Click image to enlarge. Image: Oyster Point Pharma |
More Than Water
When nasal neurostimulation hit the research scene, investigators weren’t sure if the tears generated would be quality tears. Many assumed neurostimulation would generate reflex tears, which do not have the same composition as basal tears. Others wondered if the technique would only stimulate the lacrimal glands or increase tear volume by producing aqueous without the other components needed for a healthy tear film.
Increasingly, however, the evidence suggests that nasal neurostimulation affects all parts of the LFU and increases natural, basal tearing. Studies show goblet cell degranulation, which could lead to the release of mucins, and intranasal tear neurostimulation resulted in the release of mucin to the ocular surface.5,6
Neurostimulation leads to changes in meibomian gland activity and lipid layer thickness, although a fairly long duration of use (eight minutes) was required to achieve this.7 Others found that tears collected post-neurostimulation had lipid composition equivalent to that of subjects’ basal tears.8 These early studies have been encouraging, as have the clinical results in patients treated with various forms of neurostimulation.
Nasal Neurostimulation
The first device on the market was TrueTear (Allergan), an intranasal tear stimulator. It activates the nasolacrimal reflex by delivering small electrical currents to sensory neurons of the nasal cavity, temporarily increasing tear production. The device is designed for home use for at least two minutes and up to 30 minutes per day. Studies show that regular use for six months results in a statistically significant increase in tear production and reduction in dry eye symptoms, as well as an increase in tear meniscus height.9,10
One challenge with TrueTear is the logistics of dispensing the device to patients and training them on its use. To find the “sweet spot” for delivering the electrical pulses, the tips need to be inserted as far as possible into the nose and then tilted so they contact the nerves just under the bridge of the nose (Figure 2).11
iTear (Olympic Ophthalmics ) is another handheld neuromodulation device under development. The noninvasive device is applied to the outside of the nose to activate the trigeminal parasympathetic pathway using a sonic frequency. The device is not yet FDA approved, but is currently in clinical trials. Early data suggest a decrease in symptom scores, an increase in the baseline Schirmer score and a persistent acute tear response at 180 days. Safety and subject comfort scores to date suggest a favorable risk-benefit ratio.
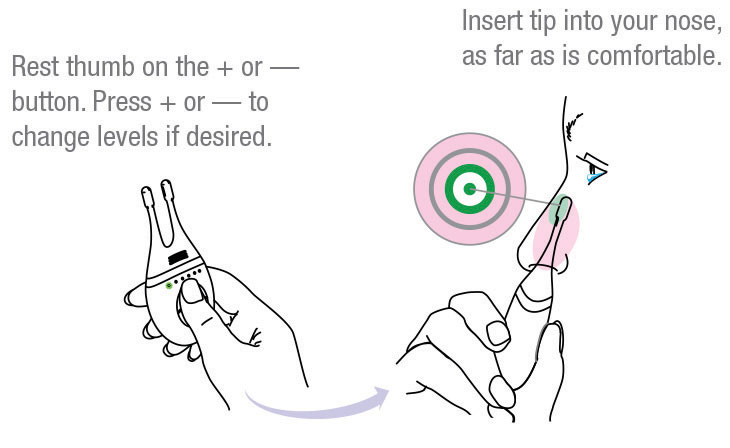 |
| Fig. 2. The TrueTear device comes with detailed instructions on its use, including this diagram to help patients place the device correctly.11 Image: Allergan |
Pharma Firsts
A nasal spray from Oyster Point Pharma is the first to tackle this pathway using a pharmacological approach. This preservative-free spray contains a nicotinic acetylcholine receptor (nAChR) agonist to stimulate the trigeminal parasympathetic pathway. nAChR receptors are found throughout the peripheral and central nervous system.
A Phase IIb study for Oyster Point’s OC-01 drug candidate enrolled 182 subjects with a diagnosis of dry eye disease. Subjects were randomized into four equal treatment groups, including placebo and three concentrations of the nasal spray (0.02%, 0.1% and 0.2%).12 All three doses produced a statistically significant improvement in dry eye signs (Schirmer score) compared with placebo at day one, week one, week two and week four. The 0.1% dose also showed a statistically significant improvement in dry eye symptoms in a controlled adverse environment challenge.
This is the first dry eye treatment study to demonstrate immediate onset of action and meet both primary and secondary pre-specified endpoints for the signs and symptoms of dry eye. Adverse events were mild and transient and, given the nasal route of delivery, the spray caused no ocular tolerability issues.
Because the nasal spray works similarly to already familiar allergy and cold and flu nasal sprays, this technology has the potential to provide nasal neurostimulation with improved patient acceptance and reduced training time. The company also expects it to be a widely available prescription product with reimbursed similar to current dry eye prescription therapies. Oyster Point hopes to begin Phase III studies this year.
Target Audience
Nasal neurostimulation is an entirely different mechanism than topical medications that target inflammation, so it has the potential to be an alternative first-line therapy in eyes before significant inflammation occurs and a complementary treatment to topical drops.
Like immunomodulator drops, nasal neurostimulation relies on patient adherence. While the route of delivery might be a stumbling block for compliance, the more immediate tearing effects may also encourage greater compliance. In addition, a novel mechanism of action is helpful for patients for whom drops are less desirable because they wear contact lenses or already use glaucoma drops multiple times per day.
Because clinical studies of these neurostimulation products have not been limited to any particular category or severity of dry eye, they may be a road worth traveling for all types of dry eye, including aqueous-deficiency, meibomian gland dysfunction and mixed etiology, whether inflammation is present or not.
Note: Dr. Karpecki consults for companies with products and services relevant to this topic.
1. van der Werf F, Baljet B, Prins M, Otto JA. Innervation of the lacrimal gland in the cynomolgous monkey: a retrograde tracing study. J Anat. 1996;188(Pt 3):591-601. 2. LeDoux MS, Zhou Q, Murphy RB, et al. Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci. 2001;42(11):2434-41. 3. Dartt DA, McCarthy DM, Mercer HJ, et al. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14(11):993-1000. 4. Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16(6):645-8. 5. Gumus K, Schuetzle KL, Pflugfelder SC, Ackerman, M. The effects of intranasal neurostimulation on tear production and clearance and conjunctival goblet cell secretion. Presented at the 8th International Conference on the Tear Film & Ocular Surface: Basic Science and Clinical Relevance, September 7-10, 2016; Montpellier, France. 6. Dieckmann G, Jamali A, Pondelis N, et al. In vivo confocal microscopy demonstrates intranasal neurostimulation-induced goblet cell alterations in patients with dry eye disease. Invest Ophthalmol Vis Sci. 2017;58(8):2694. 7. Watson M, Angjeli E, Orrick B, et al. Effect of the intranasal tear neurostimulator on meibomian glands. Invest Ophthalmol Vis Sci. 2017;58(8):4387. 8. Green KB, Kamat M, Franke M, et al. Tear total lipid concentration in patients with dry eye following intranasal neurostimulation. Invest Ophthalmol Vis Sci. 2017;58(8):2693. 9. Friedman NJ, Butron K, Robledo N, et al. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. 2016;10:795-804. 10. Orrick B, Watson M, Angjeli E, et al. Quantitation of tear production by tear meniscus height following acute use of the intranasal tear neurostimulator. Invest Ophthalmol Vis Sci. 2017;58(8):2692. 11. Allergan. Patient guide for the truetear intranasal tear neurostimulator. July 2018. 12. Oyster Point Pharma. Oyster Point announces positive results from two separate Phase 2b clinical trials of the company’s investigational treatments for dry eye disease. https://oysterpointrx.com/oyster-point-announces-positive-results-from-two-separate-phase-2b-clinical-trials-of-the-companys-investigational-treatments-for-dry-eye-disease. Accessed April 25 2019. |

