Unfortunately, DED patients today can hide in plain sight. They can be men. They can be healthy. And they can be young. Though many would argue ocular surface disease has always been complicated because of its multifactorial nature, the demographic shift only adds to its complexity.1 A survey conducted in July 2015 polled 1,000 eye care providers (ECPs) and 1,200 dry eye symptomatic adults.1 According to ECPs surveyed, 92% suspected that modern technology contributes to dry eye symptoms and the incidence of DED is increasing due to “today’s multiscreen lifestyle.”1 Perhaps the survey’s most telling statistic: 76% of ECPs reported an increase in dry eye symptoms among patients 18 to 34 years old, relative to a decade ago.1 That may not prove a definitive connection between digital device use and DED, but whatever the cause, an increase in DED is a call to action for optometrists.
This article looks at several cases of dry eye disease in younger patients, the likely instigators and how they can be successfully managed.
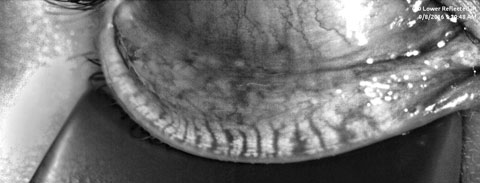 |
| Fig. 1. A patient’s meibography displays third degree gland atrophy. Click image to enlarge. |
An Indigenous Disease of Digital Natives
Whether it’s a computer or ever-present hand-held electronics, such as smartphones and tablets, patients of the Millennial generation (Americans born approximately between 1980 and 2000) are glued to digital devices for both work and play.2 In our clinic, fewer patients these days are attributing their dry eye symptoms to traditional offenders such as contact lenses, environmental factors and aging. Anecdotal evidence suggests the culprit could be digital devices.To wit, teens consume approximately nine hours of screen time every day on average.2-4 They check social media outlets as many as 100 times per day.4 With prolonged digital device use, blink performance is impacted.5 When performing a visual task, research shows basal blink rate decreases by approximately 40%.5 Furthermore, studies show that blinking while at rest is initiated by desiccation of the cornea and conjunctiva, but blink phenomenon affected by computer use appears to originate from a central neural mechanism.5 A decrease in total blink rate with near activity has long been established. However, the importance of total blink number may be less significant than the completeness of each individual blink.6 A 2013 study found that the tear film continues to be stable with decreased blink frequency as long as the blinks remain complete.6 Incomplete blinks affected tear film stability and was variable with digital device exposure.6
The Tear Film & Ocular Surface Society (TFOS) offers a handy way to encourage blinking with its “Think Blink” campaign.7 The organization suggests “every 20 minutes, close your eyelids and squeeze lightly for a count of two. Open and then look 20 feet across the room for 20 seconds.”7
In a busy practice, screening all young patients may be impossible and reap few benefits for patients or a practice. Identifying at-risk patients will streamline the diagnostic process. Consider evaluating for: presence of complaint (burn, irritation, blur increasing throughout the day), digital device time (prompting the patient to consider not only time on computers, but also tablets and smartphones), make-up application (liquid foundation, concealer, eyeliner and mascara applied inside the lash margin may pose increased obstructive risk) and medications.
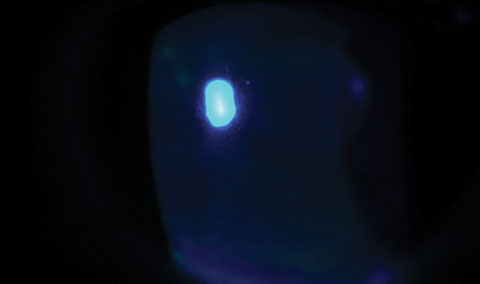 |
| Fig. 2. A slit lamp examination of the same patient revealed diffuse superficial punctate keratitis throughout the cornea. |
Testing
The ubiquitous nature of DED only complicates the lives of practitioners and necessitates additional diagnostic and point-of-care testing to identify this beneath-the-surface condition. If practitioners can no longer rely on demographics or symptomatology to point them in the right direction, additional objective testing is required. Point-of-care tests have the ability to add another piece to the diagnostic puzzle. For example, research found osmolarity accurately identified 81.3% of normal eyes and 90.7% of severe dry eye patients.2 Perhaps the more critical statistic is that osmolarity correctly identified 73.2% of mild-to-moderate patients.2 While the young, mild patient may be fairly complaint-free, the chronicity and, probably, progressive nature of DED necessitates education and, likely, treatment.While awareness of functionality in the form of blinking exercises can be coached and behavior modified, the anatomy of young patients may also show vulnerability. Meibomian gland atrophy has long been noted in aging populations.8 However, recent reports are identifying changes in a much younger demographic.9,10 Papers presented at the 2016 Academy of Optometry found gland loss in a younger subset of patients within clinical practice.9,10 Investigators found that early loss was equal between genders, unlike what we’ve seen in patients of previous generations.9 Additionally, researchers recognized that contact lens wear (orthokeratology lenses) negatively impacted gland structure in the study.9 A retrospective analysis found statistically significant differences among gender through average gland atrophy measured by the Pult score, which was 1.29 ± 0.81 for female subjects and 0.59 ± 0.58 for males.10
While additional study is required to identify risk factors for early propensity of gland atrophy, initial clinical evidence suggests that young patient populations are not immune to gland loss and tortuosity.10
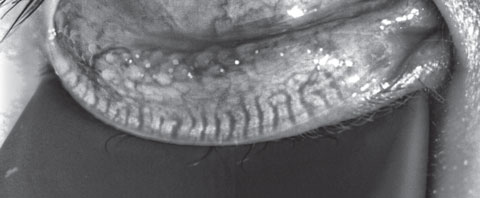 |
| Fig. 3. This patient’s meibography shows a patient with first-degree gland atrophy indicating less than 25% gland loss via the Pult scale. Click image to enlarge. |
Case #1
A 20-year-old biracial college sophomore presented with the complaint of chronic dry eye. Her symptoms included pain ranging from moderate irritation to sharp, intolerable discomfort and redness. She classified her symptoms as severe and they exacerbated with soft contact lens wear. The patient could only comfortably wear her lenses for four hours per day and often alternated the days to increase tolerability. Her ECP switched her from a bimonthly modality to daily lenses approximately six months ago. However, she did not experience significant improvement from the modification. She took no systemic medications and had no history of isotretinoin use.Prior failed treatments included artificial tears, warm compresses, lid hygiene products, antibiotic drops and steroid drops. Her entrance acuity was 20/20- OD and 20/25 OS with spectacle prescription. Her SPEED and OSDI scores were 14 and 44, respectively. Tear osmolarity was 289mOsm/L OD and 301mOsm/L OS. The Inflammadry (Rapid Pathogen Screening) testing results were negative. She had 8/8 partial blinks upon analysis and a lipid layer thickness (LLT) of 61nm OD and 58nm OS. Her tear volume, collected by phenol red thread testing, was 30mm OD and 28mm OS. Her noninvasive tear break-up time was 6.31 sec OD and 5.74 sec OS, and tear prism height was 0.35mm and 0.33mm as measured by Oculus Keratograph. Meibography revealed third-degree gland atrophy indicating between 51% and 75% gland loss via the Pult scale (Figure 1).11
Slit lamp examination revealed diffuse superficial punctate keratitis (SPK) throughout the cornea (Figure 2). While the tear prism was ample, the line of Marx, or mucocutaneous junction, had modest shift anteriorly to a point of almost transecting the meibomian gland orifices. Mild conjunctival hyperemia was noted in both eyes. Upon expression, scant meibum was visualized though the quality of the secretions was clear.
Both pharmaceutical and in-office treatment options were discussed with the patient. She elected to first pursue medical therapy with consideration for future thermal pulsation. The patient was placed on Xiidra (lifitegrast 5.0%, Shire) twice daily, a lipid-based artificial tear and provided blinking exercises with instructions for use. She was advised to remove contact lenses before using the medication and to reinsert 15 minutes after administration if needed.
Upon returning four weeks later, she noted compliance with all recommended therapies and significant resolution of symptoms. Both the SPEED and OSDI surveys improved. However, though the cornea had improved, some diffuse SPK remained in both eyes.
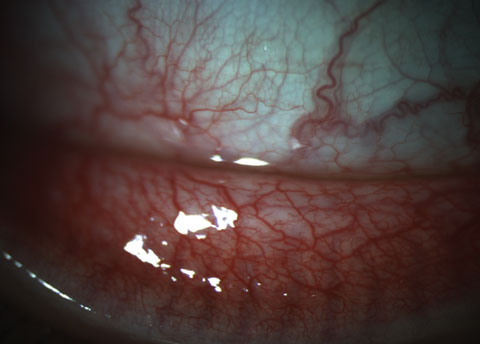 |
| Fig. 4. This patient’s 3+ papillae at the inferior fornix could be visualized at both the slit lamp as well as on meibography. Click image to enlarge. |
Case #2
A 23-year-old Asian male optometry student presented with a complaint of intermittent dryness with recent improvement in symptoms. His ECP prescribed Lotemax (loteprednol etabonate ophthalmic suspension, 0.5%, Bausch + Lomb) four times daily and artificial tears. He has worn orthokeratology lenses every evening for the past three years to improve daytime acuity secondary to moderate myopia. He felt the lenses slip some at night and reported days when the quality of vision was poor. He also had a history of hypothyroidism and daily use of levothyroxine.Prior failed treatments only include artificial tear use. Entrance acuity was 20/20 OD, OS without correction. His SPEED and OSDI scores were 9 and 31, respectively. Tear osmolarity was 296mOsm/L OD and 292mOsm/L OS. Inflammadry was faintly positive. He had 7/7 partial blinks and a lipid layer thickness of 53nm OD and 46nm OS. The patient’s tear volume was 30mm and 23mm. His noninvasive tear break-up time was 2.29 sec OD and 3.19 sec OS, and tear prism height was 0.24mm and 0.25mm. Meibography showed first-degree gland atrophy, indicating less than 25% gland loss via the Pult scale (Figure 3).
Slit lamp examination showed fine, diffuse SPK centrally OD and a mechanical keratitis OS due to the ortho-K lens because of the linear nature of the defects. We noted 3+ papillae at the inferior fornix in each eye that could be visualized at the slit lamp as well as on meibography (Figure 4). Expression of the glands was difficult and the quality of the secretion was slightly turbid in both eyes. The patient also exhibited lid wiper epitheliopathy nasally in both eyes (Figure 5).
The clinical presentation led to the diagnoses of both meibomian gland dysfunction (MGD) and acute ocular allergy. The patient began a beaded warming mask twice daily with digital massage, lipid-based artificial tears and blinking exercises. Lotemax was discontinued in favor of Bepreve (bepotastine ophthalmic solution, Bausch + Lomb) twice daily. Referral to patient’s ECP was made to reassess contact lens fit. Due to the chronic, and, likely, progressive nature of MGD, more aggressive therapies were discussed with the patient in an effort to have a greater impact on the condition and mitigate advancement.
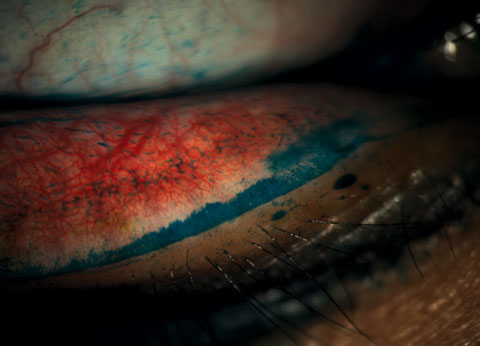 |
| Fig. 5. Staining revealed lid wiper epitheliopathy in a patient with dry eye symptoms. Click image to enlarge. |
Approach to Treatment
As cataract patients in their 90s may not get the same surgical recommendations as those in their 50s, so too will age play a role in the selection of dry eye recommendations. Doctors must consider the risk vs. the benefit. Equally, young patients likely face a long, winding path of DED. It is the doctor’s responsibility to both identify the disease and best communicate its relevance to patients. Education is key to both a fruitful and long-term relationship. Frightening the patient and taking away their contact lenses will likely drive them to another practice. On the other hand, telling the patient “everyone has dry eye” and “it’s not a big deal” will not properly convey the gravity of the condition. A balanced approach will arm the patient with the information they need to understand the disease and be compliant with recommendations. Because many of these patients will likely be in the early stages of the condition, a gradual treatment process from basic to more advanced is warranted. Consider a change in contact lens material, modality or care system, lid hygiene recommendations, blinking exercises or the appropriate artificial tear for supplementation. However, don’t rule out more advanced in-office treatment options such as thermal pulsation or intense pulsed light. Earlier, more aggressive treatment may serve the patient better in the long run.Dry eye disease was once a malady of aging women and often relegated to the lowest rung on the patient’s assessment and plan.1 Now, unfortunately, demographics of DED patients are trending younger, and doctors must broaden their minds to include this new subset.1 While new sufferers will likely be emerging, practitioners are better armed than ever before with diagnostic options and therapeutic solutions. Perhaps awareness is the greatest challenge in initiating the dialogue between doctor and patient that ultimately kick starts the journey to improvement.
Dr. Hauser is an assistant professor at Southern College of Optometry and provides clinical care for patients at TearWell: Advanced Dry Eye Treatment Center and The Eye Center. She is a consultant, speaker or board member for: Allergan, Akorn, Paragon Vision Sciences, BioTeck, BlephEx, TearScience, BioTissue, NovaBay, Lumenis, TearLab and Shire. She is founder of dryeyecoach.com.
|
1. Shire. Modern technology and a multi-screen lifestyle viewed as important factors in rising prevalence of dry eye disease. October 17, 2016. Available: www.multivu.com/players/English/7893551-shire-dry-eye-disease-awareness/. Accessed: January 19, 2017. 2. American Press Institute. Millennials’ nuanced paths to news and information. Available: www.americanpressinstitute.org/publications/reports/survey-research/millennials-paths-to-news-and-information. Accessed: January 19, 2017. 3. Lemp M, Bron A, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011 May;151(5):792-8. 4. Rideout V. The common sense census: media use by tweens and teens. Common Sense Media. Available: https://roem.ru/wp-content/uploads/2016/12/census.researchreport.pdf. Accessed: January 19, 2017. 5. Acosta M, Gallar J, Bellmonte C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Expertimental Eye Research. 1999 June;68(6):663-9. 6. Hirota M, Uozota H, Kawamorita T, et al. Effect of incomplete blinking on tear film stability. Optom Vis Sci. 2013 Jul;90(7):650-7. 7. TFOS. About the think blink campaign. Available: www.tearfilm.org/tfos-think-blink-campaign.php. Accsessed: November 19, 2016. 8. Nien C, Massei S, Lin G, et al. Effects of age dysfunction on human meibomian glands. Arch. Ophthalmol. 2011 Apr;129(4):462-9. 9. Young C, Kading D. Prevalence of meibomian gland atrophy in a pediatric population. Paper presented at The Academy of Optometry. Anaheim, CA. November, 2016. 10. Schachter S, Schachter A, Kwan J, Hom M. Gender differences of meibomian gland atrophy in younger patients. Paper presented at The Academy of Optometry. Anaheim, CA. November, 2016. 11. Pult H. Meibography in clinical practice. Ophthalmol. Times. 2012 June. Available: http://ophthalmologytimes.modernmedicine.com/news/meibography-clinical-practice. Accessed: January 19, 2017. |

