 |  |
One in three Americans is living with diabetes or pre-diabetes, according to a 2017 report from the Centers for Disease Control and Prevention.1 That’s an astonishing statistic—and a call to arms for the entire medical community.
Optometrists have mostly been able to stay on the sidelines of the fight against diabetes. Historically, general practitioners advised on prevention, endocrinologists managed patients systemically and retina specialists addressed the ocular impact. But new diagnostic tools and better treatment options are making our role in diabetes care increasingly more prominent.
The Rise of a New Treatment EraManaging patients with diabetic eye disease was considerably more difficult back when panretinal photocoagulation (PRP) was the best, and often only, option available to them. Once anti-VEGF injections were approved for age-related macular degeneration, experts naturally began wondering if the anti-angiogenic properties of these drugs could work in diabetic eye disease as well. In a word: yes. Several clinical trials have defined a new standard of care for the treatment of diabetic macular edema with intravitreal anti-VEGF injections. In 2012, the RISE and RIDE clinical trial for the treatment of DME with Lucentis (ranibizumab, Genentech) concluded that this approach was safe and effective; soon thereafter, it became an important first-line therapy for DME. In 2014 the FDA approved a second compound, Eylea (aflibercept, Regeneron) for DME, following publication of the VISTA and VIVID studies. Expanding beyond the treatment of DME, Lucentis was approved in 2017 to treat all complications of diabetic retinopathy, even in the absence of DME, following the results of RISE and RIDE. Over a 36-month period, treatment with Lucentis was found to result in a two-step reduction in the level of severity of diabetic retinopathy. Phase III results of the PANORAMA study of Eylea use in moderately severe to severe NPDR concluded that Eylea was also effective in causing a two-step reduction of the level of severity of DR in up to 80% of patients. The FDA is scheduled to comment on the approval of Eylea for the treatment of NPDR this year. |
The question we must now ask ourselves is whether early intervention is in the best interest of our patient. Complications of diabetes are the leading cause of functional vision loss in working-aged adults, and optometrists handle the majority of primary eye care in this country. Mild and moderate cases of nonproliferative diabetic retinopathy (NPDR) are typically followed in a clinical setting; however, when a patient reaches severe NPDR we should consider referring the patient to a retina specialist for an evaluation that includes fluorescein angiography to assess retinal ischemia. Factors to consider when making such a referral can include duration of diabetes, control of glucose and hemoglobin A1c values, as well as the patient’s compliance.
Severe NPDR is classified using the 4-2-1 rule: Make the diagnosis when a patient has dot-blot hemorrhages in all four retinal quadrants, venous beading in two quadrants or intraretinal microvascular abnormalities in one quadrant. The risk of progressing to proliferative diabetic retinopathy (PDR) for a patient with severe NPDR in a 12-month period is up to 50%.
Our job as optometrists is to educate our patients on the risk of visual loss secondary to diabetic retinopathy (DR). Given the results of landmark clinical trials, we are now obligated to refer patients sooner than we once may have. A recent case seen by Dr. Haynie highlights this era of change.
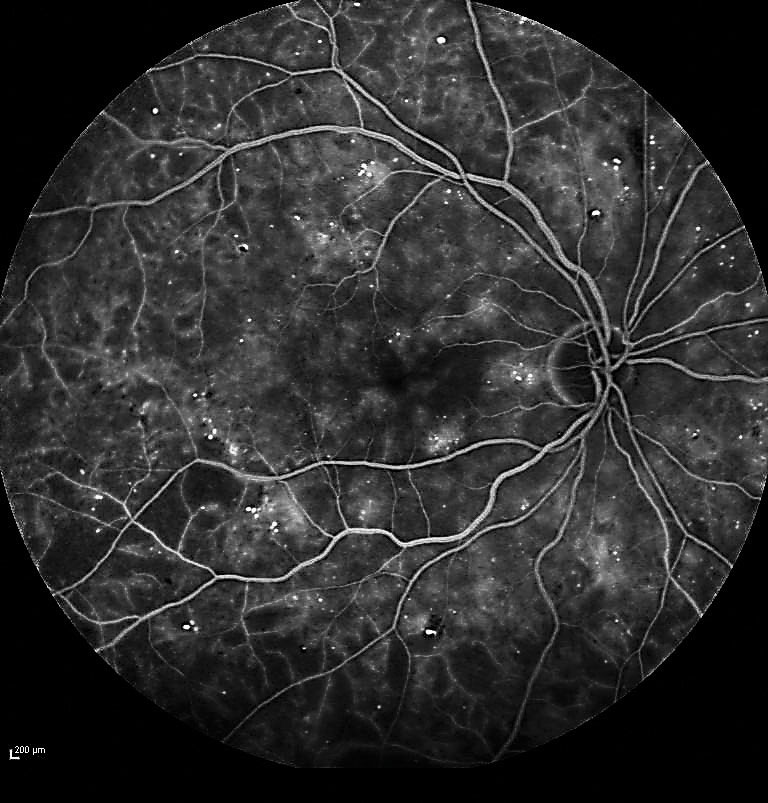 |
| Fig. 1. Fluorescein angiography confirmed severe NPDR with significant capillary nonperfusion in the temporal fundus. |
An Ounce of Prevention
Case by Dr. Haynie
A 72-year-old white female was referred for evaluation of DR. Her medical history included Type II diabetes for 17 years. Visual acuity measured 20/20 in each eye. Dilated fundus exam revealed findings consistent with severe NPDR in each eye. Fluorescein angiography was performed, confirming severe NPDR in each eye with significant capillary nonperfusion (ischemia) in the temporal fundus (Figure 1).
Following a long discussion with the patient about the risk of progression to PDR, she consented to treatment with Lucentis at 12- to 16-week intervals. Serial fluorescein angiography over two years confirms no progression of ischemia or evidence of PDR (Figure 2).
Of course, there’s no way to conclusively know that the anti-VEGF regimen is wholly responsible for the absence of progression in this case. But the results of the RISE/RIDE and PANORAMA clinical trials give us confidence in considering a referral for anti-VEGF treatment in patients with severe NPDR with or without diabetic macular edema (DME).
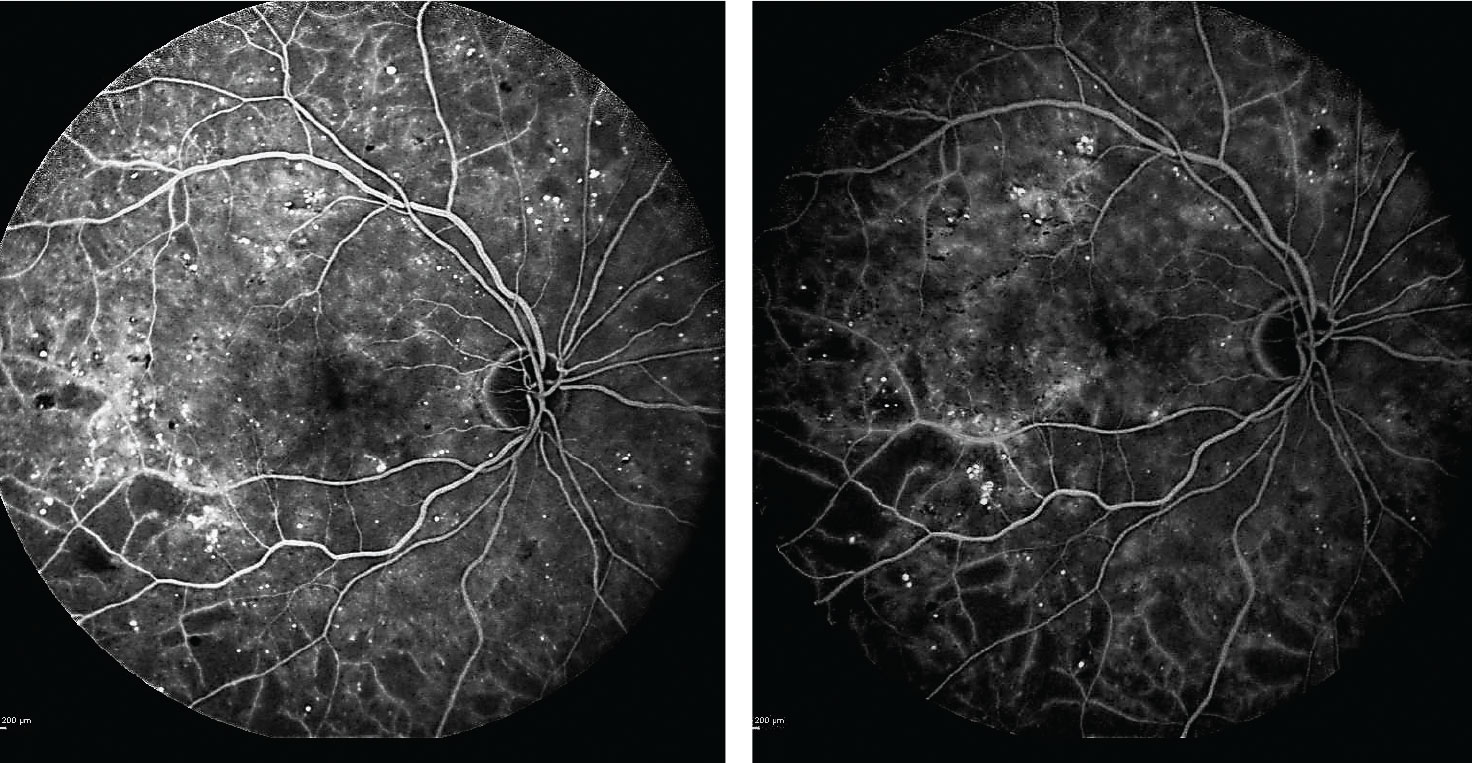 |
| Fig. 2. After beginning anti-VEGF treatments, the patient’s subsequent fluorescein angiograms at one and two years into the regimen (left and right images, respectively) confirmed no progression of ischemia or evidence of proliferative diabetic retinopathy. Click image to enlarge. |
Imaging is Imperative
Commentary by Dr. Shechtman
As optometric physicians, we need to do more than just familiarize ourselves with various treatment options for complications associated with DR, such as DME and PDR. In an evolving era of diabetes management, we also need to recognize the benefits of early intervention. The RISE/RIDE, PANORAMA and VIVID/VISTA studies, as well as DRCR.net Protocol W, have all shown benefits of anti-VEGF in reversing the severity scale of diabetic retinopathy. The recent approval of Lucentis for NPDR with or without DME indicates the beginning of a paradigm shift in the management of this condition.
We know that a patient’s level of diabetic retinopathy dictates risk for proliferative disease development, and thus the rationale for earlier intervention may be based on such risk. Although staging of severe NPDR has been characterized by the 4:2:1 rule, in my practice we feel many cases remain underestimated in terms of staging. We use ultra-widefield angiography (UFWA) in every patient who has at least moderate NPDR. In our opinion, the assessment of UWFA depicting the presence of capillary nonperfusion and subtle neovascularization may be more accurate in the staging of the disease.
Although anti-VEGF therapy may be a treatment option for severe NPDR, laser PRP is still a viable alternative, especially given compliance concerns and a patient’s socioeconomic status.
A recent expert panel concluded that PRP can be considered for certain high-risk patients with severe NPDR.2 In our practice, we have found that combination therapy with PRP and anti-VEGF agents for severe cases of DR may be a good approach for individual cases.
For optometrists, the question of when to refer is not always straightforward. The OD should consult with a local retina specialist to establish protocols for their referral patterns and treatment and management preferences. Knowing that we may in fact be able to rewrite a DR patient’s prognosis with a single well-timed referral, the conventional approach of “watchful waiting” until severity progresses to frank neovascularization may be on the wane moving forward.
Dr. Shechtman wishes to thank Wilfredo Lara, MD, for his contributions to this commentary.
1. Centers for Disease Control and Prevention. National diabetes statistics report, 2017. US Department of Health and Human Services; 2017. 2. Wong TY, Kawasaki R, Ruamviboonsuk P, et al. Guidelines on diabetic care: the international council of ophthalmology recommendations for screening, follow-up, referral and treatment based on resource settings. Ophthalmology. 2018;125(10):1608-22. |

