 |
A 38-year-old Caucasian female presented for a second opinion regarding progressive blurred vision (OU) and possible optic nerve pallor with moderate cup-to-disc ratio. Investigative historical findings elicited chronic malaise and progressive, bilateral visual decline over the course of 12 months. Further questioning revealed pronounced muscular fatigue, unsteady gait and visual decline following exercise.
Best-corrected visual acuity was 20/100 OU. The patient had pronounced horizontal jerk-type nystagmus in far lateral right and left gaze positions with noted oscillopsia. Horizontal saccades revealed a decrease in both accuracy and velocity. A dilated fundus examination revealed moderate neuroretinal rim thickness and poor perfusion of the temporal rim in each eye.
The finding of bitemporal pallor of the optic nerve prompted us to order spectral domain optical coherence tomography (SD-OCT) of the nerve fiber layer (NFL), ganglion cell complex (GCC) and formal visual fields (Humphrey 30-2). NFL and GCC were abnormally reduced in thickness bilaterally. Formal visual fields yielded a cecocentral scotoma in the right eye and scattered shallow point depressions in the left.
Evaluation
Given the above findings, the differential diagnosis included space-occupying lesion, demyelinating disease, nutritional optic neuropathy and autoimmune-related optic neuropathy. Consequently, we scheduled her for magnetic resonance imaging (MRI) of the brain and orbits with and without contrast and fat suppression. Imaging studies revealed multiple periventricular white matter (PWM) lesions.
Causes of PWM lesions are extensive but most commonly include normal (age-related) senescent changes, hypertension, focal cerebrovascular accidents, demyelination, migraine, vitamin B6 (pyridoxine) deficiency and infectious or inflammatory vasculitis.1 All ordered serologies were within normal range.
Accordingly, we referred the patient to neurology for evaluation. An additional MRI of the neck and spine was unremarkable. A lumbar puncture revealed increased levels of lymphocytes and supernumerary oligoclonal bands. Given the patient’s age, segmental pattern of optic nerve pallor, saccadic dysmetria, PWM lesions and increased levels of lymphocytes and supernumerary oligoclonal bands, she was diagnosed with primary progressive multiple sclerosis (MS) and started on Ocrevus (ocrelizumab, Genentech).
A low vision evaluation was ordered to address her specific visual needs. She was scheduled for visual evoked potential (VEP) and she will be seen bi-annually for SD-OCT of the NFL and GCC and HVF 24-2. Although the VEP was not performed during the initial consultations, we would expect a delay in latency from demyelination.
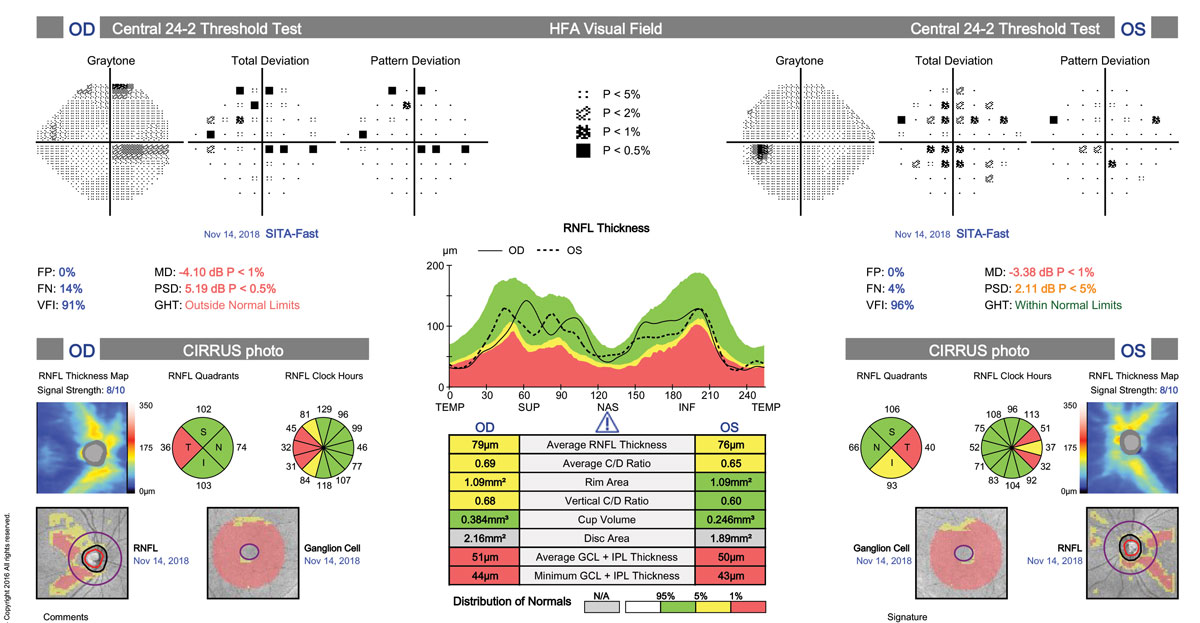 |
| Reduced NFL and GCC consistent with bitemporal pallor and VF loss can point to MS as the possible culprit. Click image to enlarge. |
Discussion
MS is the most common debilitating neurological disease found in young adults.2 The disease occurs because of an autoimmune reaction against the fatty insulation of the neurons of the central nervous system, the myelin sheath.2 During the inflammatory process, the myelin is attacked and damaged, leaving behind plaques (or lesions) detectable on MRI imaging that may or may not result in a variety of symptoms.2 While the etiology of the immune response is unknown, immunological characteristics include involvement of type 1 helper T and interleukin-17 cells which may be multifactorial.2,3,4
Practitioners should keep several ocular symptoms in mind when working with suspected MS, such as transient or chronic visual decline with or without pain, unsteady gait, bowel or bladder changes, numbness or muscular pain and trouble with speech or swallowing. Uhthoff’s phenomenon, a specific finding in demyelinating diseases, was present in our patient. Uhthoff’s phenomenon describes worsening of neurologic symptoms in MS and other demyelinating conditions when the body becomes overheated. The most common symptoms consist of transient visual blur, muscular fatigue and a feeling of light-headedness.5
MS has a highly variable course and may result in minor to major disability, depending on the sub-type present.2 There are four categories, distinguished by the disease’s course:
Relapsing-remitting MS is the most common and is characterized by flare-ups of symptoms followed by remission, during which the symptoms may improve or disappear.3,6
Secondary-progressive MS is characterized by a worsening of the disease with or without remission or plateaus of severity. This type of MS may even develop in patients with the relapsing-remitting form of the disease.5
Primary-progressive MS is characterized by symptoms that gradually worsen from the disease’s onset with no periods of remission (although there may be plateaus) and is typically more resistant to drug therapy.6
The last, and most rare, form of MS is the progressive-relapsing form, in which the disease progresses from the start with intermittent flare-ups of worsening symptoms—there are no periods of remission.6
Atypical presentations of MS-related optic nerve dysfunction include extensive bilateral optic pallor with moderate-severe bilateral vision loss.7
In our case, the patient presented with the previously-mentioned criteria, which warranted further imaging of the spine and neck and a lumbar puncture to discount neuromyelitis optica (NMO) as well as alternative infectious and inflammatory conditions. It is crucial to distinguish between MS and NMO, since the latter may respond negatively to some treatments typically indicated for MS.8 One strong characteristic of NMO is the length of spinal cord lesions on MRI.
Spinal cord lesions in NMO can span three or more continuous vertebrae, where lesions from MS are smaller and typically do not extend past two segments.9 Brain lesions in NMO often appear later and are clinically asymptomatic (with the exception of brainstem lesions), as opposed to MS where lesions occur early in the disease and serve as a piece of diagnostic criteria.9
Treatment
Numerous treatments are available for MS; however, we will focus on the two newest additions. The first FDA-approved orally administered treatment for MS is Gilenya (fingolimod, Novartis). In patients with relapsing forms of MS, fingolimod may reduce the number of relapses a patient will experience and delay disability progression.10 The mechanism is currently unknown; however, researchers believe that fingolimod reduces lymphocyte migration into the central nervous system.11
Ocrevus is the first FDA-approved drug for the treatment of primary progressive MS.12 This drug is administered via intravenous infusion.13 The drug is proposed to work by binding to CD-20-expressing B-cells in the immune system, initiating cellular and complement mediated lysis.13
MS is a complicated disease and frequent medication review is crucial for the optometrist as a member of the MS care team. The patient in this case is currently receiving Ocrevus and has been responding well to her treatments.
Suspect demyelinating disease in patients presenting with cardinal signs and symptoms such as optic nerve pallor, saccadic dysmetria, peripheral limb weakness and paresthesia. These symptoms are highly consistent with white matter lesions within the brain and/or spinal cord. Order pre- and post-contrast MRI with fluid-attenuated inverse recovery and fat suppression to substantiate demyelination.
Rapid, accurate diagnosis of MS will allow the patient to quickly receive the appropriate treatments and have the greatest chance at maintaining a high quality of life. In the setting of primary progressive MS, we recommend biannual follow-up with SD-OCT to assess the NFL and GCC as well as a HVF 24-2 and VEP.
|
1. Barkhof F, Scheltens P. Imaging of white matter lesions. Cerebrovasc Dis. 2002;13 Suppl 2:21-30. 2. National Institute of Neurological Disorders and Stroke. Multiple sclerosis: hope through research. Bethesda, Maryland: 2012. 3. Goldenberg MM. Multiple sclerosis review. P T 2012;37(3):175-84 4. Lisak R. Multiple sclerosis. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. 5.Hauser SL, Goodin DS. Multiple sclerosis and other demyelinating diseases. In: Kasper, DL, Braunwald E, Hauser SL, eds. Harrison’s Principles of Internal Medicine. 17th edition. New York: McGraw-Hill Medical; 2008:2611-21. 6. Kale N. Optic neuritis as an early sign of multiple sclerosis. Eye Brain. 2016;8:195-202. 7. Fraser CL, Davagnanam I, Radon M, Plant GT. The time course and phenotype of Uhthoff phenomenon following optic neuritis. Mult Scler. 2011:18(7):1042-4. 8. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. 9. Wingerchuk D, Lennon VA, Lucchinetti CF et al. The spectrum of neuromyelitis optica. Lancet Neurol. September 2007:6(9):805-15. 10. Occupational Health & Safety Online. FDA approves first oral drug to reduce MS relapses; September 25, 2010. ohsonline.com/articles/2010/09/25/fda-approves-first-oral-drug-to-reduce-ms-relapses.aspx. Accessed June 4, 2019. 11. Novaritis. Gilenya (fingolimod), prescribing information 2011. www.pharma.us.novaritis.com/product/pi/pdf/gilenya.pdf. Accessed on June 6, 2019. 12.FDA approves new drug to treat multiple sclerosis: first drug approved for primary progressive MS; March 29, 2017. www.fda.gov/newsevents/newsroom/pressannouncements/ucm226755.htm. Accessed June 6, 2019. 13. Genentech. Ocrevus (ocrelizumab), prescribing information. 2017. www.gene.com/download/pdf/ocrevus_prescribing.pdf. Accessed June 6, 2019. |

