 |
Best-corrected visual acuity measured 20/400 O.D. and 20/20 O.S. Confrontation visual fields showed a temporal depression in the right eye and were full to careful finger counting in the left.
The pupil exam revealed a strong relative afferent pupillary defect in the right eye. Ocular motility testing was normal.
The anterior segment exam was unremarkable. IOP measured 14mm Hg O.D. and 17mm Hg O.S.
Dilated examination of the right eye revealed the fundus changes seen in figure 2. The dilated examination of the left eye was completely normal.
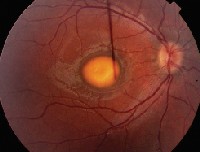 |
| 1. Fundus photo of the right eye showed this suspicious lesion involving the macula. |
1. What is the fundus lesion seen in figure 1?
a. Choroidal neovascular membrane.
b. Central serous retinopathy.
c. Choroidal nevus.
d. Choroidal melanoma.
2. What treatment did the patient likely undergo?
a. Laser.
b. Photodynamic therapy.
c. Plaque radiotherapy.
d. Surgical excision.
3. What is the most significant finding along the vascular arcades?
a. Vessel attenuation.
b. Exudate.
c. Candle-wax drippings.
d. Cotton-wool spots.
4. What caused the fundus changes shown in figure 2?
a. Branch retinal vein occlusion.
b. Diabetic retinopathy.
c. Radiation retinopathy.
d. Recurrent choroidal neovascularization.
5. How should this patient be managed?
a. Laser photocoagulation of the choroidal neovascularization.
b. Photodynamic therapy.
c. Panretinal photocoagulation.
d. Observation.
For answers to this months quiz, please look below.
Our patient had a suspicious choroidal nevus when she initially presented. Within several months, the lesion became malignant and showed evidence of growth.
Historically, enucleation had been the standard of care for treating uveal melanomas until eye-conserving methods, developed in the 1970s, came into widespread clinical use.
The Collaborative Ocular Melanoma Study (COMS) evaluated some of these different options. Specifically, COMS randomized patients who had choroidal melanomas to various treatment regiments depending on the size of the tumor, namely:
Smaller tumors (1mm to 3mm in apical height and at least 5mm in basal diameter).1 The physician decided how he or she wanted to manage the patient, as patients who had small melanomas were judged to have a low risk of dying within five years.
Medium-sized tumors (3.1mm to 8mm in apical height and 16mm or less in basal diameter).2 Patients in this group were randomized to undergo enucleation or iodine plaque radiotherapy.
Survival rates between the two groups were equal. Thus, enucleation rates in both Europe and America have dropped significantly, as more patients are being treated with more conservative eye-preserving therapies, such as radiotherapy.
Large tumors (more than 8mm in apical height and/or more than 16mm in basal diameter).3 Patients in this group were randomized into two groups: those who would undergo enucleation alone and those who would undergo external beam radiation that would be followed by enucleation.
External beam radiation did not improve survival rates compared to enucleation alone, so external beam radiation prior to enucleation is not recommended.
Our patient underwent radiotherapy with an episcleral radioactive iodine-125 plaque. The iodine-125 isotope is now the most commonly used isotope in the United States for this type of treatment. Iodine-125 has replaced older forms of plaque therapy, such as cobalt-60, ruthenium-106, iridium-192 or palladium-103 as the mainstay of plaque radiotherapy treatment.4
The iodine-125 plaque is sutured onto the sclera directly above the melanoma, then removed three to seven days later. While in place, the radioactive particles within the plaque emit radiation directly into the malignant melanoma.
Successful treatment of uveal melanomas requires levels of radiation that are higher than what the retina and optic nerve can tolerate.
Radiation retinopathy is a recognized adverse effect of plaque radiotherapy treatment. Given the option, however, patients are more likely to accept the eventual decrease in vision than undergo enucleation.
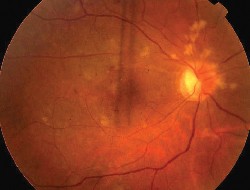 |
| 2. This is the fundus of the right eye after treatment. Note the vascular changes along the superior arcade. |
Radiation retinopathy typically occurs between six and 24 months after treatment. The endothelial cell wall lining of blood vessels is the main target of radiation-induced damage.
Microaneurysms, capillary dropout and neovascularization are all characteristics of radiation-induced retinopathy. Macular edema is also common. The degree of visual loss depends on the extent of vessel damage and non-perfusion.
The appearance of radiation retinopathy can be similar to that of hypertensive or diabetic retinopathy. The appearance of the fellow eye (i.e., no similar findings), along with the patients history, allows for the correct diagnosis.
The vascular changes seen in our patient are all complications of radiation retinopathy. Our patient developed cotton-wool spots six months after treatment, and hemorrhages and vessel sclerosis about one year after treatment. She also developed extensive capillary non-perfusion along the superior arcade and retinal hemorrhages along the inferior arcade.
With this amount of ischemia, our patient subsequently developed neovascularization as well as a vitreous hemorrhage. As a result, panretinal photocoagulation became necessary.
Her melanoma has shown an involutional response to the radiation and has remained stable. The patient continues to be checked biannually for systemic metastasis and is followed every four months by her ocular oncologist.
Her vision eventually dropped to 3/200 in her right eye and has remained at that level.
Retina Quiz Answers: 1) d; 2) c; 3) d; 4) c; 5) d.
Samantha Ward, O.D., an optometric resident at the Bascom Palmer Eye Institute in Miami, co-authored this case.
1. Mortality in patients with small choroidal melanoma. COMS report No. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 1997 Jul;115(7):886-93.
2. Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS report No. 18. Arch Ophthalmol 2001 Jul;119(7):969-82.
3. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, II: initial mortality findings. COMS report No. 10. Am J Ophthalmol 1998 Jun;125(6):779-96.
4. Nag S, Quivey JM, Earle JD, et al. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003 Jun 1;56(2):544-55.

