Treating pain has long been a difficult task for practitioners, particularly with so many drugs on the market. Further complicating this topic is the subjective nature of pain, as there is no objective way to assess the true level of a patient’s pain. Several pain scales are available, including a numerical scale, a color scale and a facial grimace scale; however, these are somewhat subjective as a patient may underestimate or overestimate their pain intensity.
The two main types of pain are nociceptive and neuropathic pain. Nociceptive pain is the normal processing of pain, where pain is in response to signals conducted from a normal, intact nervous system. Neuropathic pain occurs when signals are sent to a damaged nervous system.1,2 In quantifying pain using the numeric scale, mild pain is 1-3, moderate pain is 4-6, and severe pain is 7-10.
Pain that presents in the optometric patient will generally be acute and nociceptive in nature and will be effectively treated with non-opioids and opioid medications. Painful ocular conditions include trauma, infection, uveitis corneal abrasions or ulcers or scleritis. In choosing an agent to manage acute pain, use one appropriate for the level and type of pain (i.e., inflammatory vs. non-inflammatory). It should have a fairly rapid onset, an extended half-life, while causing the fewest drug interactions and side effects possible.3,4 With or without a DEA license, optometrists can effectively treat most patients with eye pain with currently available over-the-counter and prescription agents.
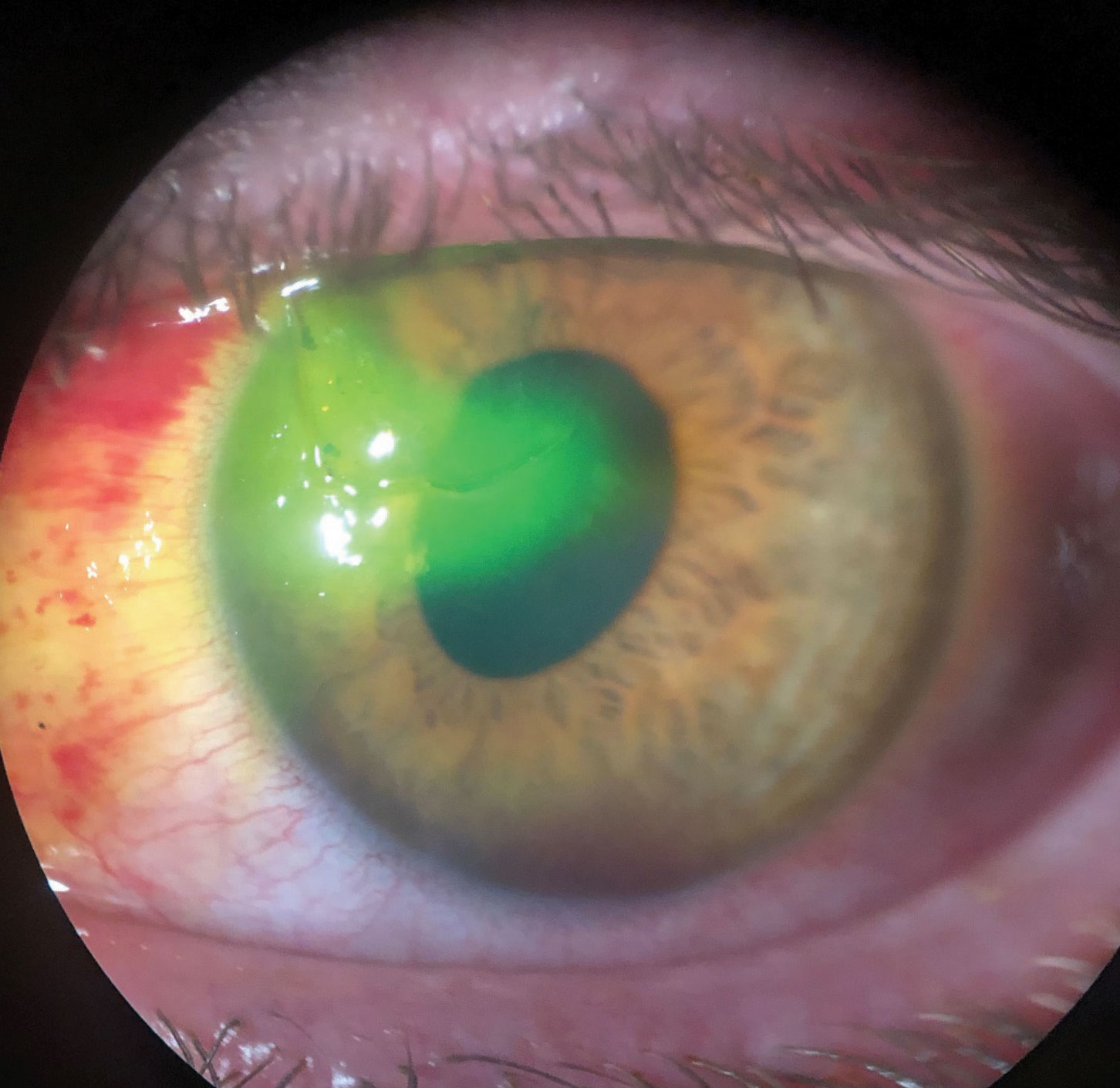 |
| This patient suffered a traumatic corneal laceration after a mishap with a screwdriver. Note the sodium fluorescein being pulled into the stroma and the oblong pupil. Click image to enlarge. |
Mild to Moderate Pain
Tylenol (acetaminophen, Johnson & Johnson) may cause rather mild inhibition of prostaglandin synthesis. It has no useful anti-inflammatory potential.6 Make sure your patients choose a product that has acetaminophen as a single ingredient, as many over-the-counter products contain acetaminophen with unnecessary ingredients, such as antihistamines, decongestants and expectorants. While the FDA still lists the maximum daily dose at 4,000mg, some practitioners prefer to limit that to closer to 3,000mg since antihistamines can cause unwanted dry eyes and drowsiness.9
Side effects associated with acetaminophen are fairly mild as long as patients follow the appropriate dosing guidelines. Potential adverse effects include hepatotoxicity. In fact, in the United States, acetaminophen is one of the leading causes of acute liver failure requiring a transplant.7 The Food and Drug Administratiosuggests that patients who consume more than three alcoholic beverages per day are at an increased risk for hepatotoxicity. Usually, hepatotoxicity is unintentional, where patients do not follow appropriate safe dosing guidelines or where they mix acetaminophen with other pain medications that also contain acetaminophen (i.e., Vicodin, Percocet).8 In 2011, the FDA asked manufacturers of prescription products that contain acetaminophen, to reformulate all products to contain no more than 325mg of the drug per dosage unit.9 Acetaminophen is generally safe in pregnancy and breastfeeding.2,3,9
Mild-to-moderate and Moderate Pain Meds
|
Nonsteroidal anti-inflammatory drugs (NSAIDs) include a variety of agents that differ in structure as well as in side effect potential. In the general pain management of an optometric patient, Advil (ibuprofen, Pfizer) or Motrin (ibuprofen, Johnson & Johnson) and Aleve (naproxen sodium, Bayer) are typically the only agents needed to effectively control mild-to-moderate pain, and they have the added benefit of having anti-inflammatory potential, which may be beneficial in patients with inflammatory eye pain. Aspirin is also an NSAID; however, its use is generally limited to A low dose as a cardiac protectant in select patients, mainly because of side effects like hypersensitivity, tinnitus, and gastrointestinal erosion in all patients, and Reye syndrome in susceptible children.10
Mechanistically, traditional NSAIDs reversibly inhibit cyclooxygenase-1 and 2 (COX-1 and 2) enzymes, which decrease the formation of prostaglandin precursors.10-12 This prostaglandin inhibition gives us a decrease in pain, inflammation and fever; however, it also produces the gastrointestinal and renal side effects that are associated with NSAIDs. Additionally, aspirin irreversibly inhibits thromboxane A2, which is what results in the cardiac protection.10-12 NSAIDs do not and instead result in a potential increase in cardiovascular risk.10-12 The dosing of NSAIDs varies greatly; however, the anti-inflammatory potential of these agents is generally only seen at the higher end of the dosing range (Table 2).3,11
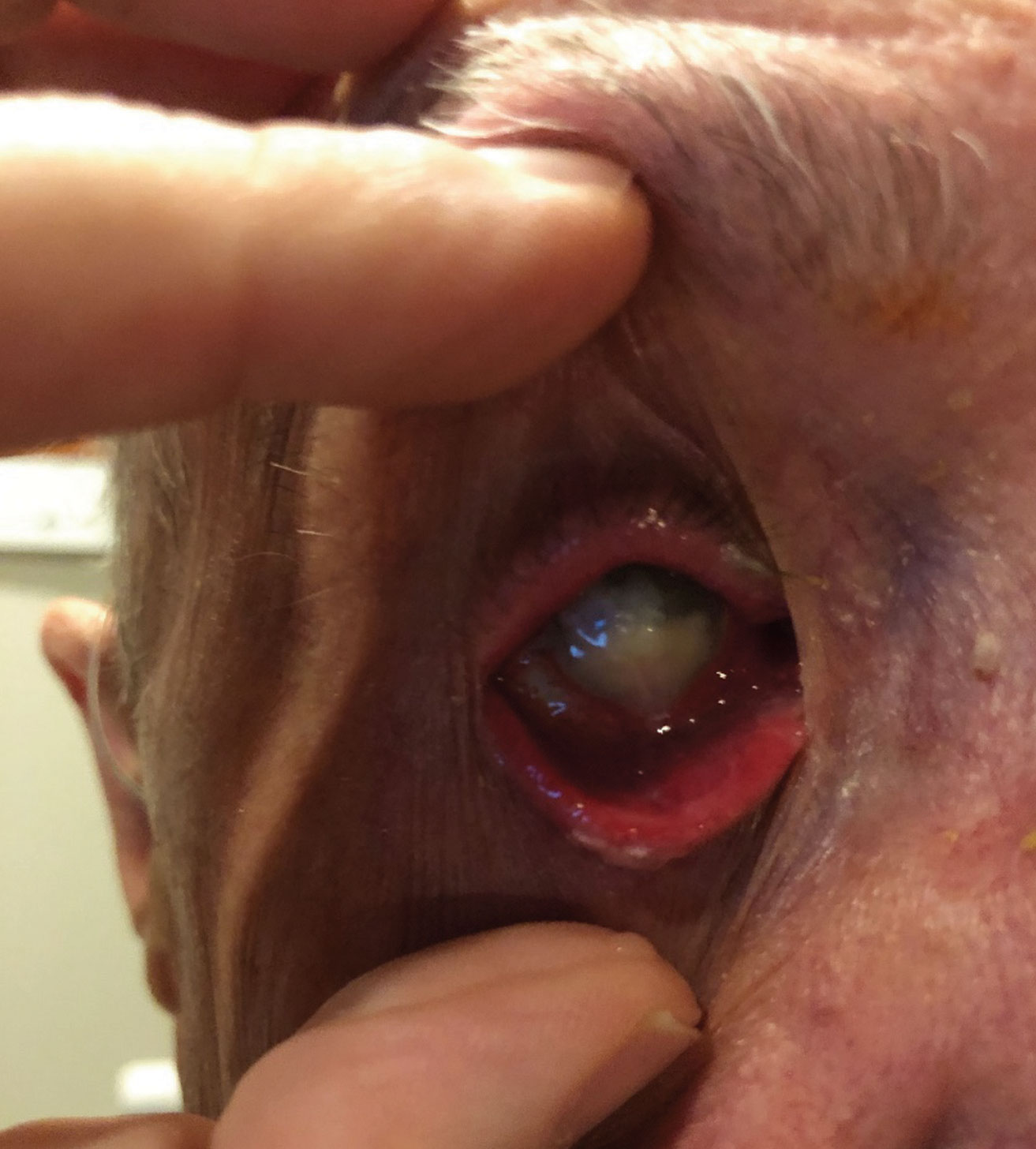 |
| This patient presented with severe pain secondary to endophthalmitis that he developed after being noncompliant following a corneal transplant. Click image to enlarge. |
The side effects of traditional NSAIDs are well documented. Because NSAIDs reversibly inhibit prostaglandins, platelet aggregation effects may increase the cardiovascular risk, particularly when used long-term or in those with underlying cardiovascular/cerebrovascular disease. Additional toxicities include gastrointestinal (GI) erosion, renal vasoconstriction, hypersensitivity (rash, respiratory, anaphylaxis) and hematologic abnormalities.12-15 Upper GI complications are the most common manifestation in the gut and sometimes concomitant use of a proton-pump inhibitor, such as omeprazole or esomeprazole may be warranted. Additionally, all NSAIDs should be taken with food to add a layer of protection between the drug and the lining of the gut. Food is not a fool-proof way to protect against GI issues, as the prostaglandin inhibition is not a topical effect but a systemic effect.3,11-15
Renal vasoconstriction is a side effect that is more likely to occur in patients who are dehydrated, elderly, on angiotensin converting enzyme inhibitors or angiotensin receptor blockers, or in those with heart failure.30 More than one risk factor increases the risk of vasoconstriction and acute renal failure. Additionally, NSAIDs can increase blood pressure, as these agents increase sodium and water retention in the blood stream in some patients. Optometrists should be aware of this blood pressure increase; however, it is not necessarily a predictable outcome in every patient who takes a NSAID. Lastly, hypersensitivity reactions may occur in patients taking NSAIDs, exacerbating asthma symptoms or causing a hypersensitivity reaction in patients without asthma. In general, if a patient is allergic to aspirin then there is a chance that they will also react to other NSAIDs. Due to the side effect potential of these agents, it is generally recommended that patients use NSAIDs in lower doses and for the shortest duration of time to limit adverse effects.3,10,11-15
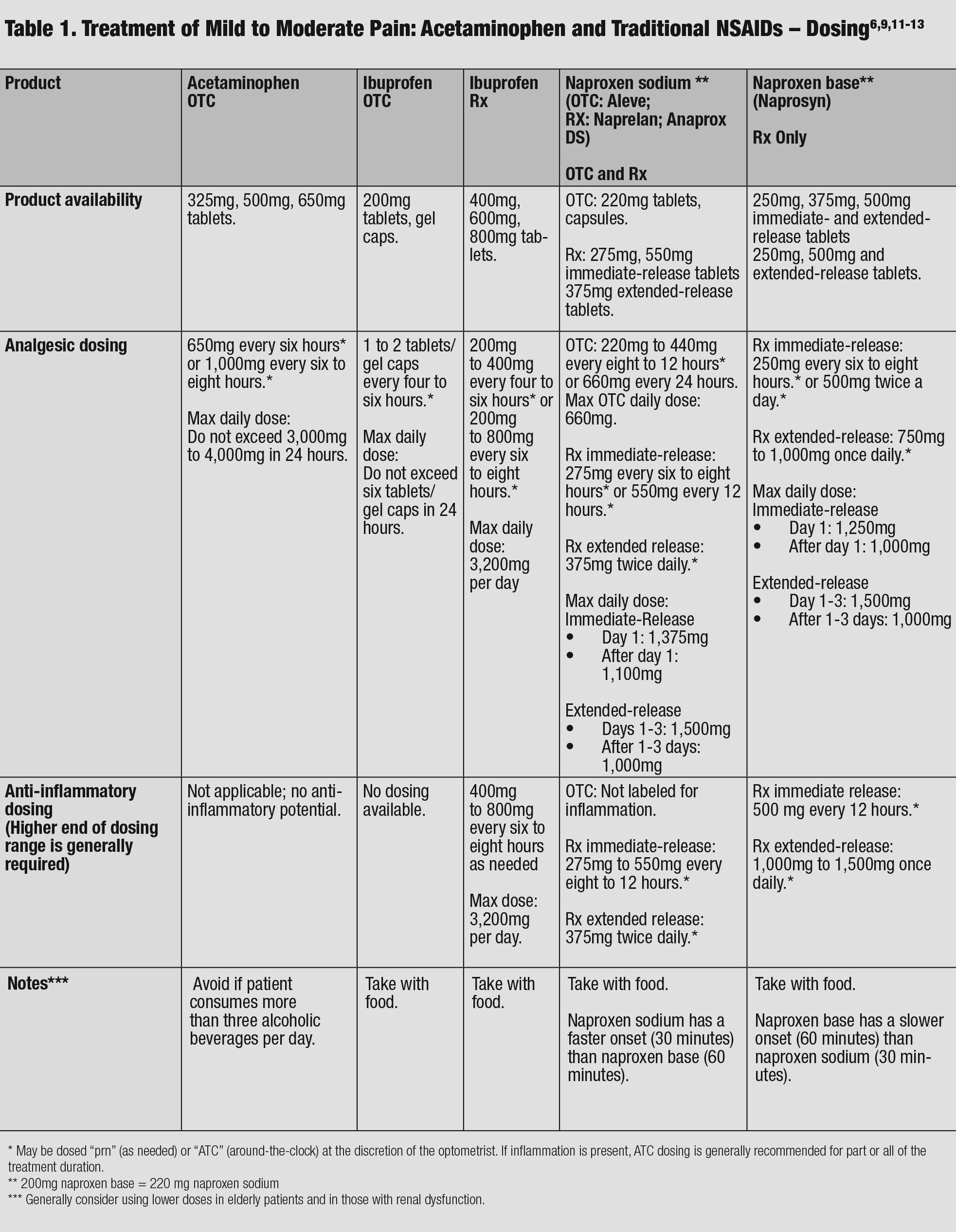 |
| Table 1. Treatment of Mild to Moderate Pain: Acetaminophen and Traditional NSAIDs – Dosing. Click table to enlarge. |
NSAIDs are generally contraindicated in pregnancy but may be acceptable in breastfeeding women, depending upon the dose of the drug, the age of the infant and the number of times the infant is nursing. Optometrists are encouraged to consult the patient’s pharmacist or pediatrician with questions regarding the safety of drugs in breastfeeding.11 In patients currently taking Warfarin (coumadin, Bristol-Myers Squibb) or other anti-platelets or anti-coagulants, care must be taken to avoid bleeding Warfarin interacts with NSAIDs by more than one mechanism, so ODs and patients must be in contact with the practitioners that prescribed Warfarin.3,10-15
A cyclo-oxygenase 2 (COX-2) specific agent such as Celebrex (celecoxib, Pfizer) or a COX-2 selective agent such as Mobic (meloxicam, Boehringer Ingelheim Pharmaceuticals) may also be reasonable choices in patients with mild-to-moderate pain. Traditional NSAIDs inhibit both COX-1 and COX-2 enzymes in the arachidonic acid cascade. Researchers have discovered that inhibition of COX-2 enzymes is primarily responsible for two things: anti-inflammatory potential and cardiovascular risk. This means that COX-2 agents are better for chronic, inflammatory pain; however, their risk for cardiovascular complications is generally considered to be more likely to occur than with a traditional NSAID.
Regarding the gastrointestinal tract, the COX-2 agents are thought to be marginally less toxic to the gastric mucosa when compared to the traditional NSAIDs.3,12,17 Additionally, it is thought that COX-2 specific and selective agents are less effective in pain management (particularly acute pain), as COX-1 enzyme is also involved in the transmission of pain. Celebrex and meloxicam are therefore not generally recommended in acute pain scenarios like those seen in optometric patients.3,12,15-17
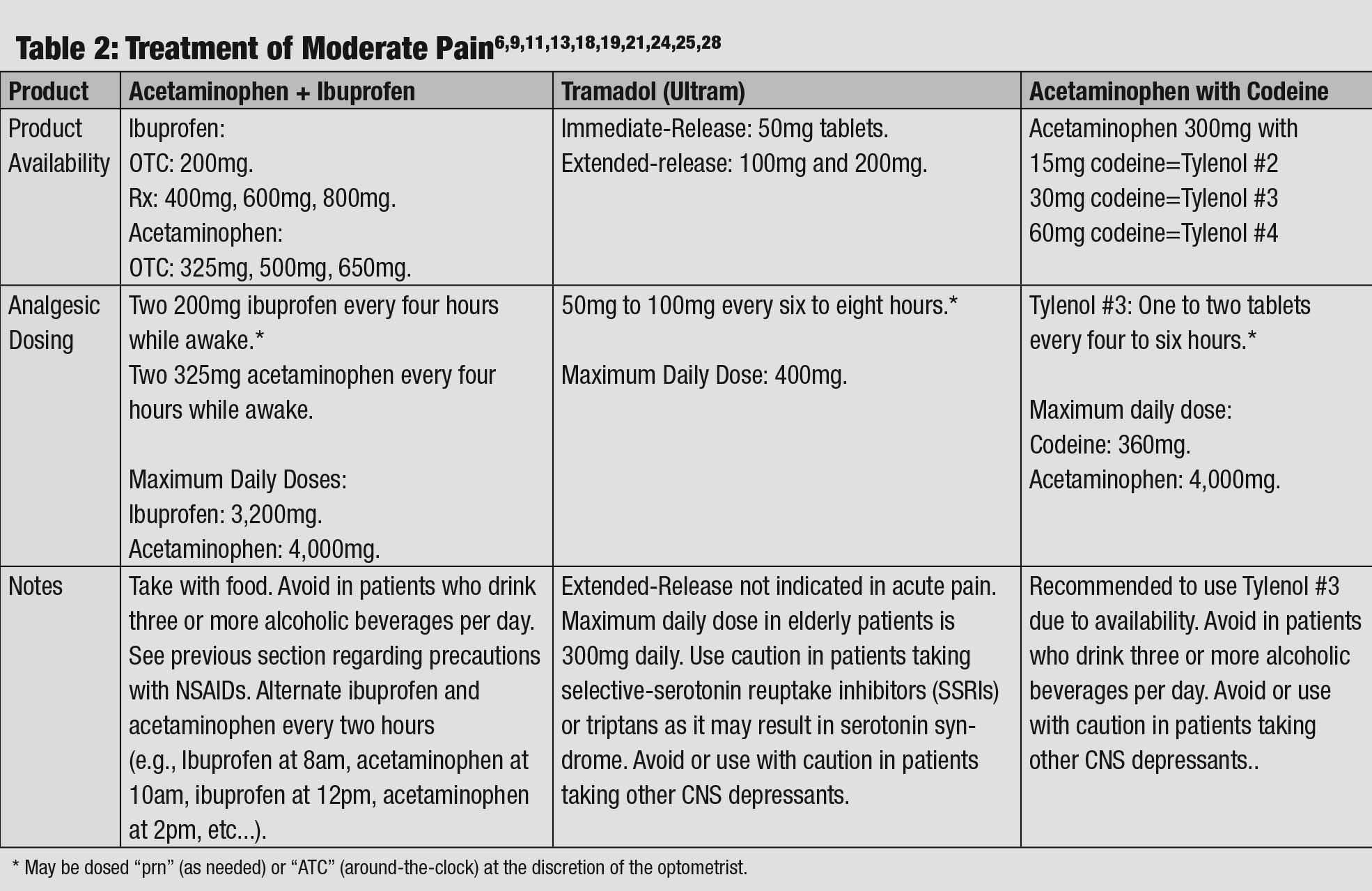 |
| Table 2: Treatment of Moderate Pain. Click table to enlarge. |
Moderate Pain
Acute pain that is a four to six on the numeric scale may be appropriately treated with higher doses of acetaminophen, NSAIDs or acetaminophen plus NSAID.31,32 If those drug options are insufficient, practitioners with a DEA license may consider a Schedule IV controlled substance such as Ultram (tramadol, Johnson & Johnson), or a Schedule III controlled substance such as Tylenol-Codine No. 3 (acetaminophen with codeine, Johnson & Johnson). Acetaminophen plus ibuprofen dosed alternately have proven to control pain better than acetaminophen or ibuprofen alone.6,9,13,18,19 The typical dosing regimen can be referred to as “two and two every four hours,” which means that two acetaminophen are given every four hours alternating with two ibuprofen every four hours while awake (Table 2).6,9,13,18,19
Ultram is another option in the management of moderate pain. It has a dual mechanism of action, as it plugs into the mu opioid receptor as an agonist and it also inhibits the reuptake of serotonin and norepinephrine, which results in a relative increase in both of these neurotransmitters.15,21-23 Ultram became a Schedule IV controlled substance in 2014, because at higher doses it can cause euphoria. Additionally, it is sometimes used by opioid abusers to mitigate withdrawal symptoms when other opioids are not available. In general, Ultram is fairly well tolerated and is less likely to cause sedation when compared with other opioid analgesics, due to the dual mechanism of action.15,21-23 Typical side effects include mild sedation, mild constipation and dizziness. The FDA requires Ultram to have a “black box warning” cautioning patients and prescribers about potential respiratory depression and drug interactions. This drug is contraindicated in pregnancy and breastfeeding and should be avoided in patients with allergic reactions to other opioid analgesics and in patients with a substance abuse history.20,21-23
Tylenol-Codeine No. 3 is a commonly used combination opioid in the management of moderate pain. It is a relatively weak opioid, and it is generally understood that 200mg of oral codeine is equivalent to 30mg of oral morphine. Mechanistically, opioids such as codeine plug into mu, kappa and delta receptors in the central nervous system (CNS), producing inhibition of ascending pain pathways.24,25
Tylenol-Codeine No. 3 is available in 15mg, 30mg and 60mg tablets; however, it is generally recommended to use the product that contains 30mg of codeine with 300mg of Tylenol-Codeine No. 3, as it is the most widely available at pharmacies. The most common side effects include nausea, vomiting, constipation, sedation, miosis, euphoria, respiratory depression and dizziness.
When an opioid is used in the management of ocular pain, ODs may recommend that the patient take measures to manage the constipation, such as Peri Colace (senna + docusate sodium, Avrio health), which contains both a stimulant laxative and a stool softener, but with a typical course of only three to five days, this may not be necessary.26 Acetaminophen with codeine is generally contraindicated in pregnancy and breastfeeding, and it should be avoided in combination with other CNS depressants due to additive CNS and respiratory depression. Codeine products should not be used in patients with a Type-1 hypersensitivity reaction to codeine or morphine derivatives, nor in patients with a substance abuse history.3,4,7,16,20,24,25
Severe Pain Meds
|
Severe Pain
The highest degree of ocular pain—a seven to 10 on the numeric scale—may be treated with a stronger opioid analgesic, such as hydrocodone with acetaminophen. These options include Vicodin (hydrocodone bitartrate and acetaminophen, Abbott) and Norco (hydrocodone bitartrate and acetaminophen, Allergan). This requires a DEA license that allows optometrists to write for this Schedule II narcotic. Any hydrocodone with acetaminophen is generally contraindicated in pregnancy and breastfeeding, and it should be avoided in combination with other CNS depressants due to additive CNS and respiratory depression. These products should not be used in patients with a Type 1 hypersensitivity reaction to codeine or morphine derivatives, nor in patients with a substance abuse history.1,7,20,24,27,28
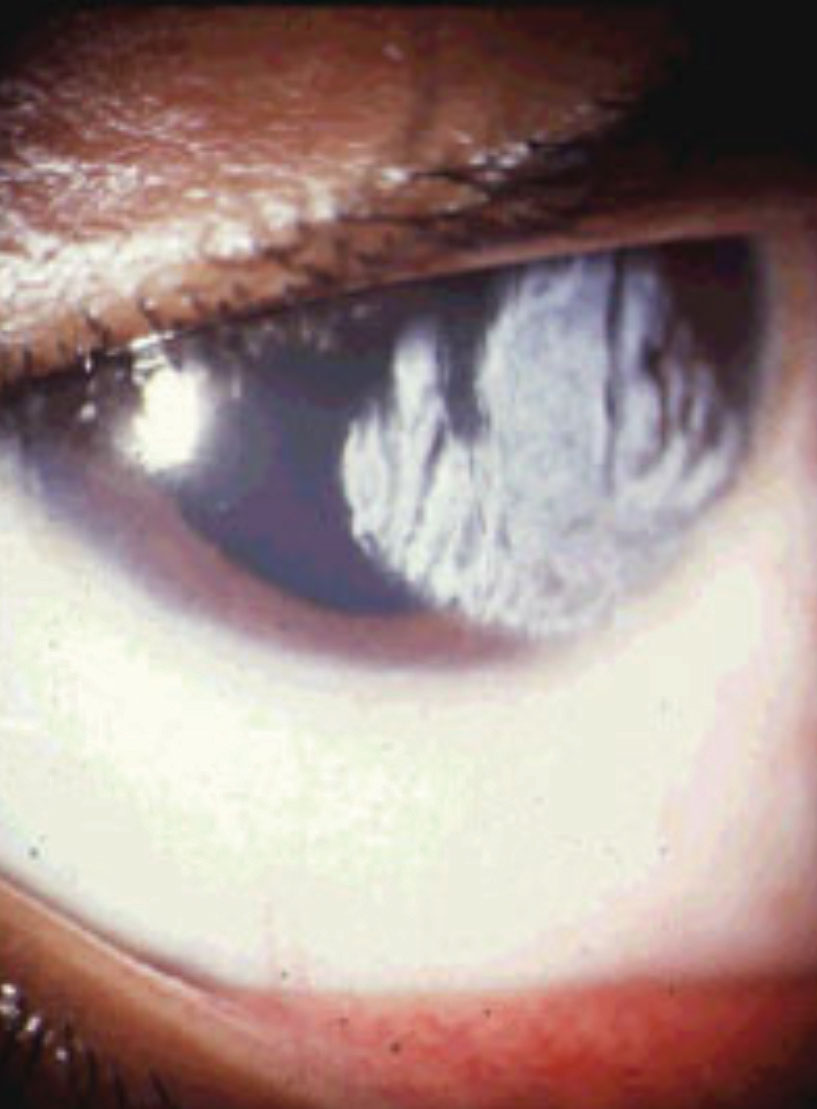 |
| This patient’s corneal burn developed following an incident with a curling iron. They were treated with a bandage contact lens and antibiotic steroids as well as proper oral threapy based on their reported pain severity. |
With or without a DEA license, optometrists still ought to familiarize themselves with the pharmacologic agents available for pain treatment. No patient should needlessly suffer, so appropriate choices need to be carefully evaluated on an individual basis. With the recent emphasis on the “opioid crisis,” it is required of many optometrists with prescribing privileges for systemic medications to enroll in the prescription drug monitoring program (PDMP).29 This free website covers several dozen states, and it lists every controlled substance, every practitioner that has written a prescription for a controlled substance and every pharmacy that has filled a prescription for a controlled substance. This valuable resource helps every medical practitioner determine what controlled substances a patient is taking, which is helpful in lessening the chance of drug interactions, as well as helps determine if a patient has a prescription drug use habit.
A practitioner’s best plan is the old sports metaphor that says “the best offense is a good defense,” where the offensive plan is to arm oneself with knowledge. In particular, this should include education regarding medication choices, legislative changes to controlled substances, and tools available to monitor an individual patient’s use of controlled substances. Most patients do not present with drug-seeking behaviors; however, consistent education and vigilance by the optometric practitioner will ensure that appropriate choices are made for individual diagnoses and patients. In doing so, no patient suffers with acute ocular pain.
Dr. Offerdahl-McGowan is an assistant professor of biomedicine at Salus University.
P. Scout McGowan is a pre-med student at Delaware Valley University
Dr. Caldwell is an ocular disease consultant in Duncansville, PA, and a past president of the Pennsylvania Optometric Association.
Marissa Mangoni is a pre-med student at Delaware Valley University.
1. Kusuhara S, Fukushima Y, Ogura S, et al. Pathophysiology of diabetic retinopathy: The old and the new. Diabetes Metab J. 2018;42:364-376. 2. McCulloch D, Robertson R. Pathogenesis of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus. Accessed March 8, 2019. 3. Gupta N, Mansoor S, Sharma A, et al. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7:4-10. 4. Wang W, Lo A. Diabetic retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018;19:E1816. 5. Miao C, Zhang G, Xie Z, Chang J. MicroRNAs in the pathogenesis of type 2 diabetes: new research progress and future direction. Can J Physiol Pharmacol. 2018;96(2):103-12. 6. Kaidonis G, Gillies M, Abhary S, et al. A single-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasian patients. Acta Diabetol. 2016;53:643-50. 7. McCulloch D, Hayward R. Screening for type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/screening-for-type-2-diabetes-mellitus. Accessed March 7, 2019. 8. Wexler D. Initial management of blood glucose in adults with type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/initial-management-of-blood-glucose-in-adults-with-type-2-diabetes-mellitus. March 21, 2019. Accessed May 20, 2019. 9. McCulloch D. Oveview of medical care in adults with diabetes mellitus. UpToDate. www.uptodate.com/contents/overview-of-medical-care-in-adults-with-diabetes-mellitus. March 21, 2019. Accessed May 20, 2019. 10. Colagiuri S, Cull C, Holman R, UKPDS Group. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: U.K. prospective diabetes study 61. Diabetes Care 2002;25:1410. 11. Simó-Servat O, Simó R, Hernández C. Circulating biomarkers of diabetic retinopathy: an overview based on physiopathology. J Diabetes Res. 2016;2016:5263798. 12. Fonseca V. New developments in diabetes management: medications of the 21st century. Clin Thera. 2014;36(4):477-84. 13. Dugan K, DeSantis A. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/glucagon-like-peptide-1-receptor-agonists-for-the-treatment-of-type-2-diabetes-mellitus. January 14, 2019. Accessed March 8, 2019. 14. Dugan K, DeSantis A. Dipeptidyl peptidase-4 (DPP-4) inhibitors for the treatment of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/dipeptidyl-peptidase-4-dpp-4-inhibitors-for-the-treatment-of-type-2-diabetes-mellitus. January 22, 2019. Accessed March 10, 2019. 15. De Santis A. Sodium-glucose co-transporter 2 inhibitors for the treatment of type 2 diabetes mellitus. UpToDate. www.uptodate.com/contents/sodium-glucose-co-transporter-2-inhibitors-for-the-treatment-of-hyperglycemia-in-type-2-diabetes-mellitus. December 10, 2018. Accessed March 10, 2019. 16. Mohamed Q, Gillies M, Wong T: Management of diabetic retinopathy: a systematic review. JAMA 2007;298(8):902-16. 17. Chew E, Davis M, Danis R, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the ACCORD eye study. Ophthalmology. 2014;121(12): 2443-51. 18. Gao L, Xin Z, Yuan M, et al. High prevalence of diabetic retinopathy in diabetic patients concomitant with metabolic syndrome. PLoS ONE. 2016;11(1):e0145293. 19. Chen Y, Wang Y, Zhao D, et al. High prevalence of lower extremity peripheral artery disease in type 2 diabetes patients with proliferative diabetic retinopathy. PLoS One. 2015 Mar; 10(3):e0122022. 20. Patrick JS, Tyler ME. Fundus Photography overview. In: Ophthalmic Photography: Retinal Photography, Angiography, and Electronic Imaging, 2nd ed. New York, NY: Butterworth-Heinemann Medical; 2001. 21. Bethke W. The devil’s in the distant details. Rev of Ophthalmol. 2014;20(8):26-28. 22. Kiss S. Going ultra-wide. Retina Today. 2018;13(10)46-8. 23. Brown K, Sewell JM, Trempe C, et al. Comparison of image-assisted versus traditional fundus examination. Eye and Brain. 2013;2013(5):1-8. 24. Wessel M, Aaker G, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785-91. 25. Silva P, Cavallerano J, Haddad N, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over four years. Ophthalmol. 2015;122(5):949-56. 26. Oliver C, Schwartz S. Ultra-widefield fluorescein angiography. In: Arevalo JF, ed. Retinal Angiography and Optical Coherence Tomography, 1st ed. New York, NY: Springer Science and Business Media. 2009:407-17. 27. Reddy S, Hu A, Schwartz SD. Ultra-wide field fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9-14. 28. Muquit M, Marcellino G, Henson D, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophtalmol. 2013;91(3):251-8. 29. Cunha-Vaz J, Coscas G. Diagnosis of macular edema. Ophtahlmologica. 2010;224(suppl 1):2-7. 30. de Carlo TE, Chin AT, Bonini-Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35(11):2364-70. 31. Takase N, Nozaki M, Kato A, et al. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377-83. 32. Choi W, Waheed NK, Moult EM, et al. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina. 2017;37(1):11-21. |

