 |
A 77-year-old Caucasian female glaucoma patient presented with complaints of gradually decreased visual clarity secondary to cataracts over the past two years. It was starting to negatively impact her quality of life, and it was time to proceed with cataract surgery.
This patient presented to me more than a decade earlier with advanced glaucoma as a new patient. She has been an ideal patient over the years, vigilant and compliant with her medications. As is the case with many long-standing glaucoma patients, she has had varied issues with ocular surface disease, exacerbated by topical anti-glaucoma medications. Following the pattern for such cases, we’ve modified medications over the years to provide her with reasonable comfort, as well as therapeutic efficacy in stemming the progression of the glaucoma.
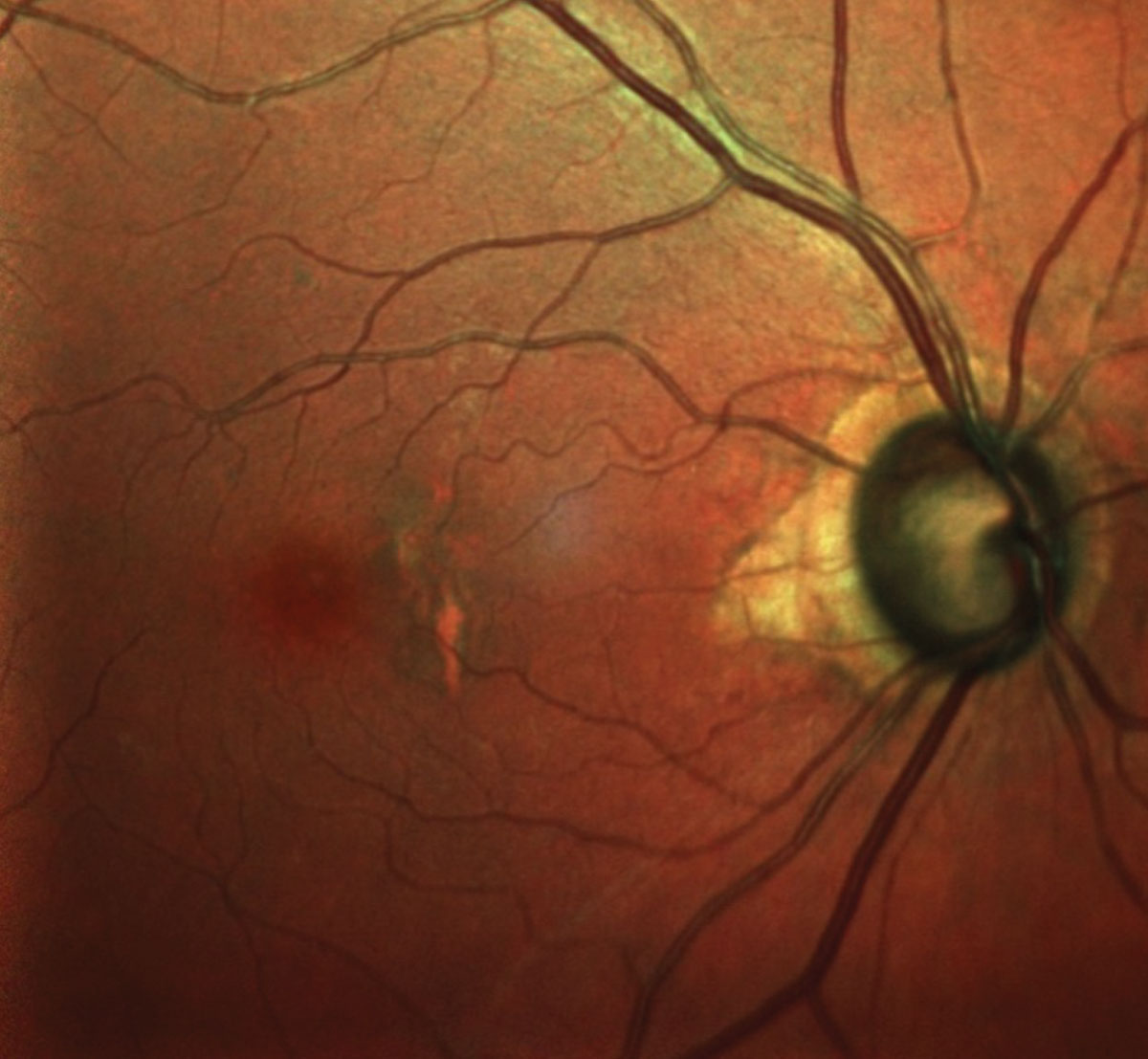 |
| The patient’s right eye, shows advanced glaucomatous damage and macular changes consistent with her age. Click image to enlarge. |
Evaluation
During her preoperative work up, we first had to establish that her glaucoma was stable, and her ocular surface in good enough shape to undergo the planned bilateral cataract extractions. At this time, her systemic medications included only lisinopril and Prilosec (omeprazole, Procter & Gamble). Also at this time, best-corrected visual acuities were 20/50-OD and 20/40-2 OS, both decreasing significantly under glare conditions. The anterior segment was characterized by mild inferior superficial punctate keratitis (SPK), mild episcleral injection and a thin tear prism OU. This presentation was consistent with her anterior segment characteristics for the past several years and was deemed acceptable for cataract surgery.
Applanation tensions at this visit were 12mm Hg OD and 13mm Hg OS, which were consistent long-term with her target pressures and at a level where there had been no progression of her glaucoma. Treated intraocular pressures (IOPs) were achieved by using Travatan Z (travoprost, Novartis) HS OU and 0.5% timolol QAM OU. She had been on this regimen for the past four and a half years. Gonioscopy demonstrated open angles in both eyes with the scleral spur visible in most quadrants, and previous ultrasonic biomicroscopy demonstrated a slight plateau approach in both eyes.
Through dilated pupils, her crystalline lenses were characterized by 2+ cortical spoking OU, mostly off the visual axis, as well as 2+ nuclear sclerosis OU and early PSC formation in the right eye more than the left. The onset of the posterior subcapsular cataracts in the previous six months contributed greatly to her decreased quality of life.
Her optic nerves were average sized and characterized by 0.8 x 0.9 cupping OD and 0.85 x 0.9 cupping OS. The temporal neuroretinal rims were thinned OU, and there was associated zone beta peripapillary atrophy temporally in both eyes.
In the past decade, the peripapillary atrophy had gradually increased, but fortunately we were able to stave off further neuroretinal rim damage by constant vigilance and medication modification when warranted. These optic nerve appearances were consistent with what she initially presented with as a new patient many years ago.
Previous visual fields demonstrated bilateral arcuate field defects encroaching fixation in the left eye more than the right, and were last completed six months prior. Heidelberg retina tomography and optical coherence tomography performed on the preoperative visit demonstrated no change as compared with previous scans in three important areas: the neuroretinal rim, the perioptic retinal nerve fiber layer circle scans, and the macular ganglion cell analyses. Structurally, these images showed she was stable, and previous field studies demonstrated her functional stability.
Her macular evaluations were characterized by mild retinal pigment epithelium mottling in both eyes, consistent with her age, and no evidence of subretinal neovascular membrane formation. Her retinal vasculature was essentially unremarkable, as were the findings of her peripheral retinal evaluations.
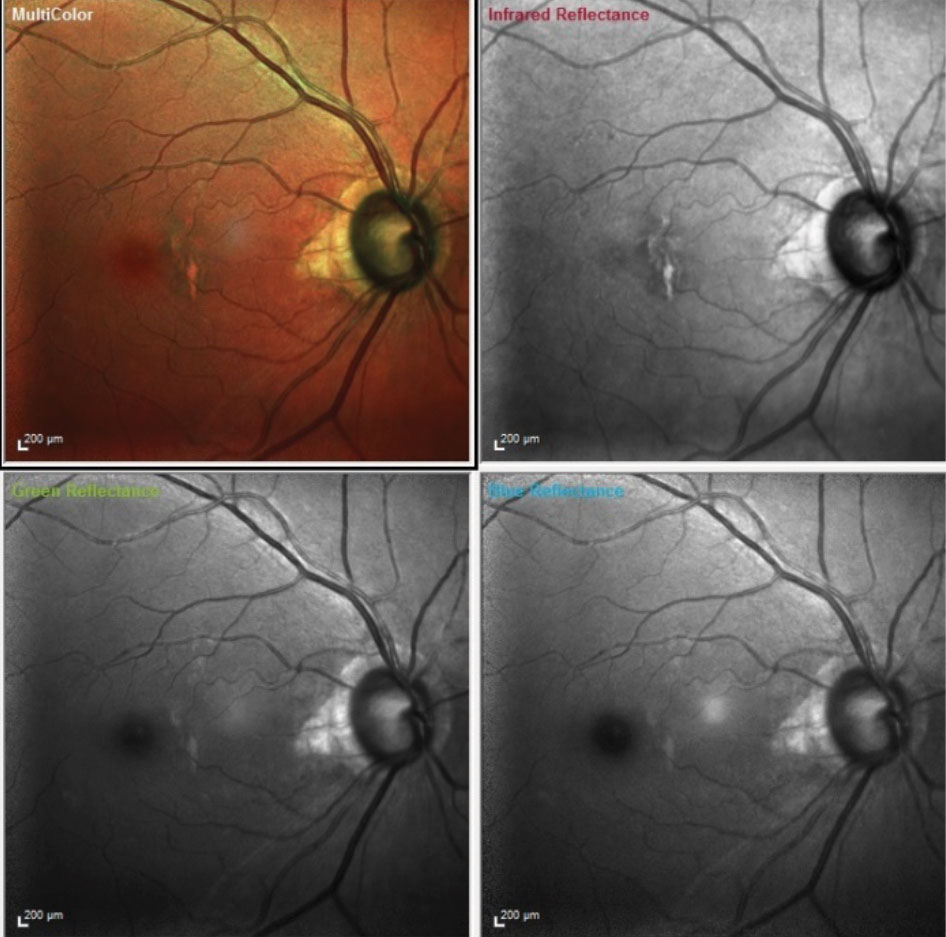 |
A multi color composite image of the patients right eye is obtained through the use of three different wavelength laser images stacked on top of each other to provide a composite of the fundus. Each image highlights different layers of the retina. Infrared laser highlights the choroid, the green laser highlights the intraretinal layers and blue highlights the retinal nerve fiber layer. These help us see strucnuances that occur structurally in the retina in these various diseases. |
Discussion
In evaluating preoperative cataract patients, make sure glaucoma patients are as well controlled as possible—and that ocular surface issues are addressed preoperatively. This patient was stable. The fact that she has been a compliant patient helps, as the addition of postoperative medications can complicate an already full regimen.Post-cataract surgery medications will vary depending upon the surgeon, but typically include an antibiotic, nonsteroidal anti-inflammatory drugs (NSAIDs), and a steroid. For the particular surgeon this patient was sent to, the standard postoperative regimen includes Besivance (besifloxacin, Bausch + Lomb) BID x 2 weeks, Prolensa (bromfenac, Bausch + Lomb) QD x 4 weeks and Durezol (difuprednate, Novartis) BID for two weeks and tapered as inflammation subsides. With the use of fluroquinolones and NSAIDs postoperatively, the course of care is pretty much the same whichever drugs are used. However, steroids can have significant, and problematic, postoperative differences. In general, the postoperative steroid regimen will vary in one of three ways, and typically include Durezol, Pred Forte (prednisolone, Allergan) or Lotemax (loteprednol etabonate, Bausch + Lomb). They each have good and bad qualities for glaucoma patient undergoing cataract extraction.
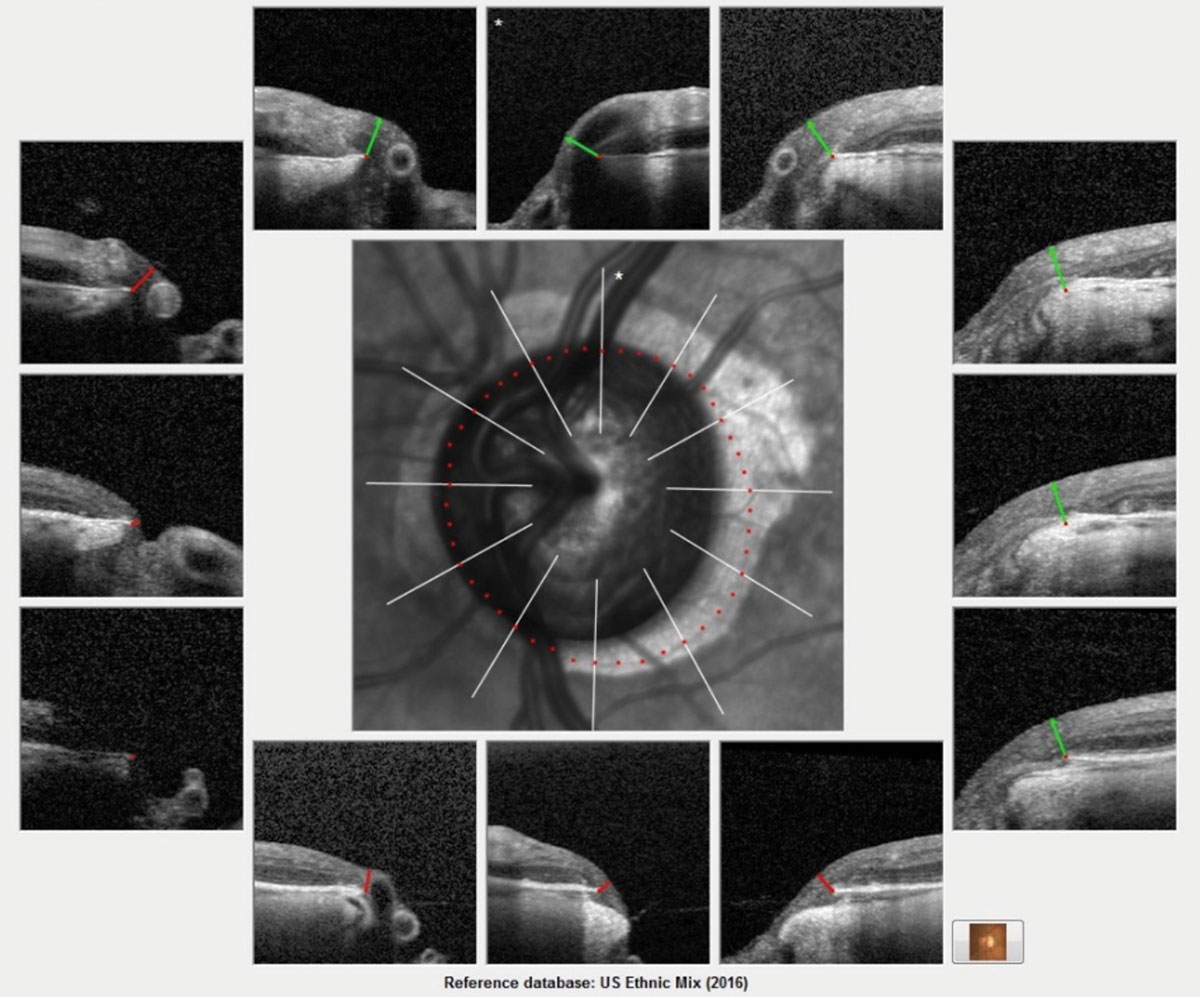 |
The Bruch’s membrane overview printout for this patient shows advanced glaucomatous damage. This image helps get a ‘global’ picture of the neuroretinal rim. The actual scanning of the optic nerve obtains many more images and is more clinically detailed. Each scan measures the ganglion cells inside the optic nerve, specifically in the neuroretinal rim. A reduction in the thickness reading is consistent with progressive ganglion cell loss. Click image to enlarge. |
Therapeutic Nuances
Glaucoma patients have a higher tendency to develop IOP spikes following the administration of topical steroids, and this must be kept in mind when seeing these patients postoperatively. In general, those steroids that are most likely to reduce inflammation are more likely to elevate IOP, especially in glaucoma patients. So, the dichotomy between side effects of IOP elevation, and anti inflammatory effects, must be taken into consideration when managing glaucoma patients postoperatively.
Postoperative steroid dosing varies; with Durezol typically being dosed BID, Pred Forte QID and Lotemax QID. Given the tendency for Durezol to elevate IOP, this particular patient was given the following postoperative regimen: Besivance BID, Pred Forte QID, and Prolensa (bromfenac, Bausch + Lomb) QD. However, the patient was unable to obtain the Pred Forte, and was told to pick up a sample at the surgeon’s office. When she arrived, she was given Durezol and her instruction sheet was not adjusted accordingly and so she took the steroid QID in the operated eye.
On her day five postoperative follow up visit, her visual acuity uncorrected was 20/30+, with minimal central corneal edema, negative Siedel sign, mild incisional bullous keratopathy, a quiet chamber with trace particle and trace cell only, and an IOP of 31mm Hg. She was using the all her medications appropriately.
When IOP spikes develop postoperatively in glaucoma patients, especially in patients with advanced disease (such as this patient), it is imperative to reduce IOP to prevent further damage to the already fragile neuroretinal rim. This can be accomplished by adding more pressure-reducing agents or reducing the offending steroid’s dosing. Keep in mind, too, the postoperative inflammation must also be managed, which does require a steroid.
How can an optometrist balance these somewhat contradictory needs? In general, I prefer to use the fewest medications possible, so I would reduce the steroid.
By choosing the best steroid for the job initially, optometrists can mitigate steroids’ postoperative side effects (assuming proper compliance and dosing). Personally, I insist the patient use Pred Forte, partially because it has less IOP elevation potential than some steroids and more anti-inflammatory effects than others. But I also have a more important, more practical reason.
With Durezol (on a BID dosing schedule) if the patient experiences an IOP spike, you really only have two options to titrate down the dosage; either use the Durezol QD or stop it completely. Usually, cutting down the Durezol to once daily has minimal effect in reducing IOP, as even the QD dosing can keep pressure elevated. And stopping it in the immediate postoperative period is not a good idea either when considering inflammatory control.
On the other end of the spectrum is the option of Lotemax QID. While it won’t usually elevate IOP as much as Durezol, it is a weak anti-inflammatory agent, and, in my experience, tapering Lotemax too quickly can result in protracted anterior segment inflammation.
Pred Forte is my preferred postoperative steroid because it has good anti-inflammatory efficacy when dosed QID and can be decreased to TID, BID or QD to mitigate any intraocular pressure rises, In other words, clinicians have three options before discontinuing the medication, whereas with Durezol they only have a QD option, with minimal effect on lowering IOP.
Doctors can quickly find themselves painted into a corner, trying to manage inflammation and IOP spikes secondary to topical steroids. By choosing a steroid that supports numerous dosing schedules, you have more options at hand to control both issues.

