 |
Drusen, along with pigment mottling, are the earliest clinical signs and a characteristic hallmark of age-related macular degeneration (AMD).1,2 This is an important clinical finding, as AMD ranks globally as the third leading cause of blindness after cataracts and glaucoma.2 Since AMD is chronic and progressive, early identification is imperative in delaying the course of the disease and referring for prompt treatment when necessary to preserve visual integrity.2
However, drusen are also known to occur in younger eyes without AMD. This discussion of the unique pathophysiology and histological changes in AMD, and how it differs from healthy, non-pathological drusen deposition, can help you differentiate the two.
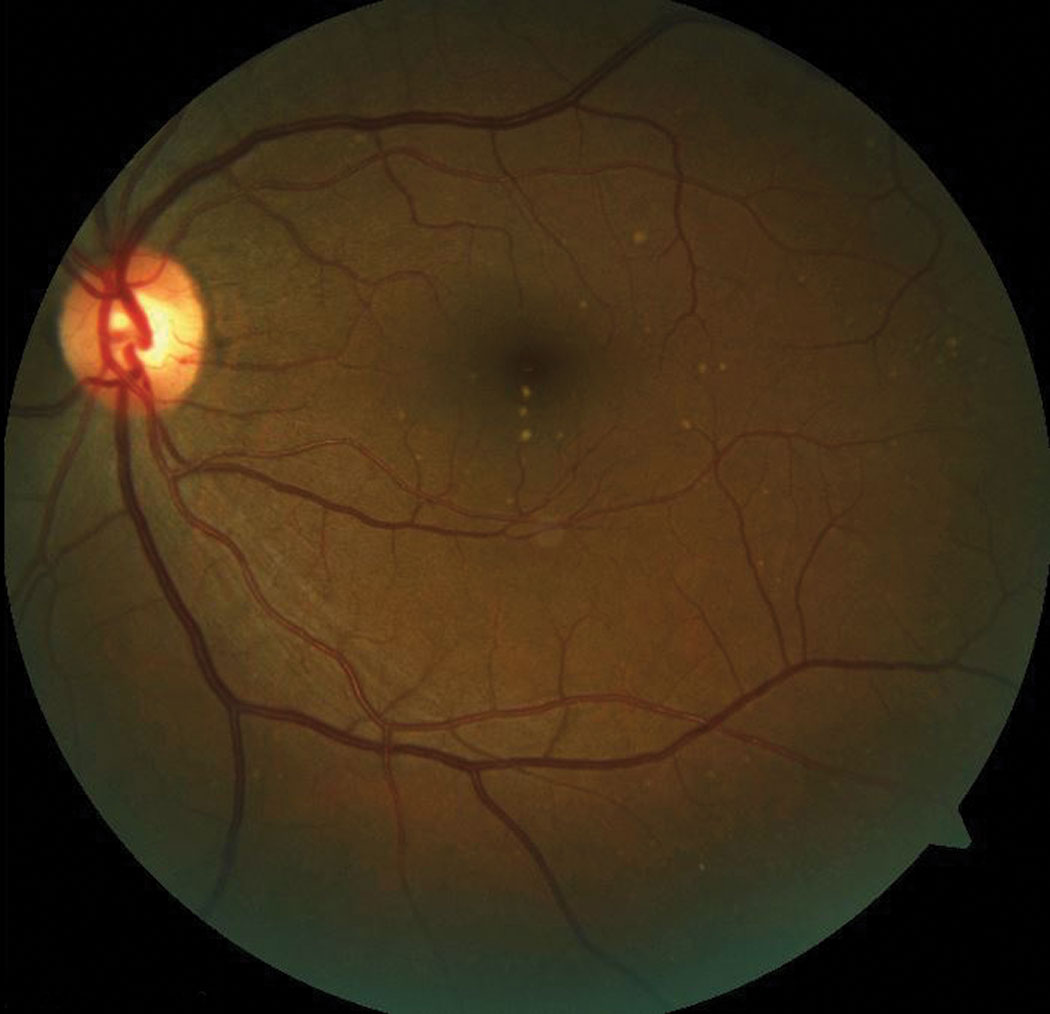 |
| This patient has subretinal drusen deposits in the macular region. Click image to enlarge. |
Debris Buildup
Drusen are extracellular deposits of debris and waste products that accumulate at the level of Bruch’s membrane below the retinal pigment epithelium (RPE).1 Clinically, they appear as focal, whitish yellow excrescences beneath the retina, often of variable size and number.3 Despite significant advancement within the past several years in understanding the exact pathogenesis of AMD, the mechanism of drusen formation is not yet fully defined.1
Drusen deposits are made up of various proteins, polysaccharides, glycosaminoglycans and lipid, amyloid and complement factors that arise from inflammatory and immune responses to RPE cell damage.1,2 This damage is made evident as a result of continuous light exposure at the RPE/Bruch’s membrane interface and subsequent formation of reactive oxygen formation and oxidative stress.
Drusen may also become deposited from the choroidal vasculature and systemic circulation.1 The accumulation of RPE waste products, among other types of cellular debris, results in the thickening of Bruch’s membrane and decreased permeability. This influences the diffusion of nutrients and waste products between the RPE and choroidal bloodstream, leading to dysfunction.1,3 Later in the disease, this dysfunction is made visible clinically as drusen.2,3
Drusen are also found in the periphery of normal eyes without AMD, with a few modifications in its makeup compared with degenerative macular drusen. While both are made up of several types of proteins, research shows crystallins are more abundant in AMD.1 Histologically, hard drusen are amorphous, eosinophilic structures that are PAS positive and are much more compact than soft drusen. They are also associated with overlying RPE defects, whereas soft drusen are not. However, they may exhibit overlying RPE pigmentary changes.
Compared with the peripheral retina, the macula contains a greater number and density of photoreceptors and RPE cells, which contributes to the sharp visual acuity in that region. With normal aging, macular rod density declines by up to 30% while the number of photoreceptors, with the exception of severe AMD cases, remains stable. Light exposure over time and the accumulation of oxidative damage leads to reactive oxygen species formation and the impairment of outer segment phagocytosis in this area by the RPE cells. Waste products then accumulate within the RPE and lead to deteriorating vision.
Drusen also attract increased inflammatory and vasoactive stimuli that can lead to progressive and more advanced signs of AMD, such as hemorrhaging and neovascular formation.3
Consistency is Key
Two main clinical classifications of drusen exist: hard and soft.
Hard drusen are smaller in size, nodular and appear clinically as discrete yellow spots.1,2 Hard drusen are found in different ages and populations and generally are not a risk factor for AMD.1,2,4 In fact, small, yellow lesions with sharply distinct margins are common in younger adults and reportedly a highly hereditary trait.5
Soft drusen are larger and more diffuse. It is the presence of numerous and confluent soft drusen that is considered a major risk factor in the development and progression of AMD.
Imaging Add-ons
Diagnosis of drusen is most commonly made through clinical exam and often with the help of color fundus photography. More recently, optical coherence tomography (OCT) has been helpful in the diagnosis and progression of drusen and additional changes related to AMD. Both hard and soft drusen appear as RPE elevations that are homogenous and convex structures. Progression is observed on OCT through changes in size as well as overlying pigment. It is also helpful in identifying additional AMD-related conditions, such as the presence of choroidal neovascularization, hemorrhage or pigment epithelial detachments.6
A Moving Target
In addition to variable mechanisms of formation and consistency, drusen can come and go. They are not always a steady clinical finding, as one study found between 20% and 34% of drusen disappear within a five to seven year span.7 Researchers speculate growing drusen indicate a functioning RPE capable of secreting debris. Once the drusen are large enough, however, the RPE cells either migrate or die, cutting off the drusen’s supply. This allows the clearing process to catch up, causing the drusen to disappear.8 The variable presentation from visit to visit this process creates can lead to significant diagnostic confusion.
The pathological mechanisms for AMD are multifactorial and complex in nature, despite intense progress in research. As AMD is a leading cause of vision loss with limited treatment options, it is important for clinicians to identify the clinical risk factors for the development and progression of the disease as early as possible. Certain interventions, such as vitamin supplements, are beneficial only in earlier stages of the disease, warranting careful monitoring to reduce risk of debilitating vision loss.
1. Crabb JW. The Proteomics of Drusen. CSH Perspectives in Medicine. 2014;4(7):1-14. 2. Gheorghe A, et al. Age-related macular degeneration. Romanian Journal of Ophthalmology. 2015(59);2:74-77. 3. Algvere PV, et al. Drusen maculopathy: a risk factor for visual deterioration. Acta Ophthalmology. 2016;94:427-433. 4. De Jong PTVM. Elusive drusen and changing terminology of AMD. Eye. 2019;32:904-914. 5. Pederson HR, et al. Multimodal imaging of small hard retinal drusen in young healthy adults. Br J Ophthalmol. 2018;102:146-152. 6. Schmidt-Erfurth U, Klimscha S, Waldstein SM, Bogunović H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye (Lond). 2017;31(1):26-44. 7. Curcio CA. Soft drusen in age-related macular degeneration: biology and targeting via the oil spill strategies. Invest Ophthalmol Vis Sci. 2018 Oct;59:AMD160-181. 8. Pilgrim MG, Lengyel I, Lanzirotti A, et al. Sub-retinal pigment epithelial deposition of drusen components including hydroxyapatite in a primary cell culture model. Invest Ophthalmol Vis Sci. 2017;58:708-19. |

