The standard of care for keratoconus (KCN) has changed very little over the years. Approximately 10% to 20% of patients will eventually require some form of keratoplasty due to the progressive nature of KCN.1 In addition, most patients, whether before or after undergoing grafting surgery, will require gas permeable (GP) lenses to reduce optical aberrations and achieve adequate functional vision.
However, the status quo is undergoing revolutionary modifications due to the recent FDA approval of corneal collagen crosslinking (CXL). Currently indicated for treating progressive keratoconus and post-surgical ectasia, CXL represents the most significant treatment paradigm change for this population.
Current clinical data shows CXL is safe and highly efficacious in halting or slowing the progression of ectatic diseases—a clinical task that was previously thought to be impossible.1-3 In addition, although KCN prevalence is often cited as around 1/2,000, recent epidemiological evidence shows it may be as high as 1/375 in certain populations.4-5 As such, the impact of CXL may be more far-reaching than anticipated.
This article discusses the benefits of CXL, candidacy selection, treatment techniques, potential complications and how we can best incorporate this innovative medical treatment into our care for keratectasia patients.
The Science Behind CXL
KCN typically presents during early puberty and progresses until the fourth decade of life.6 The relative stability seen in most patients beyond this stage of life is possibly a byproduct of the cumulative aging-associated crosslinking observed in all connective tissues, including the cornea. Researchers have also noted a similar phenomenon of corneal stiffening in diabetic patients where additional tissue crosslinking is purported as a result of glycation.7
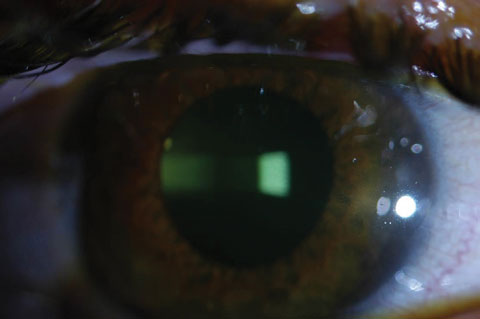 | |
 | Fig. 1. At left, the saturation of riboflavin is visible in the corneal stroma after riboflavin loading (two-minute intervals for 30 minutes). Above, the clinician checks for aqueous riboflavin staining after 30 minutes of riboflavin loading. |
The principle of artificially accelerating formation of corneal crosslinks was first explored in Germany at the University of Dresden in the late 1990s. After epithelial removal, the researchers saturated corneal stroma with riboflavin (a photosensitizer) and then exposed the treatment zone to an ultraviolet-A light with wavelength of 365nm. This spectral emission was specifically selected since riboflavin absorption peaks at approximately 366nm. The photochemical reactions led to the production of singlet oxygen-free radicals and activation of the lysyl oxidase enzymatic pathway, which induce formation of new covalent bonds to sufficiently enhance tissue stiffness that slows down or arrests KCN advancement.8
During this initial investigation, the researchers observed corneal stability in 100% of the CXL-treated study eyes. They were also pleasantly surprised by a 2.01D of flattening with maximal keratotometry readings in 70% of patients enrolled in the study.8 This discovery opened the door to a new era of ophthalmic CXL applications in keratoconus and ectasia patients and set the stage to explore various CXL techniques.
Approval and Implications
CXL received two treatment labels from the Food and Drug Administration (FDA) in 2016: progressive keratoconus and corneal ectasia following refractive surgery. The FDA’s decision on CXL was unique in that its approval was granted as a specific drug-device combination, which includes the KXL UV system and Photrexa family of riboflavin solution (Avedro). This reflects the fact that the multicenter study data submitted for FDA review was derived solely from this single treatment platform using a traditional epithelium-off technique. Although Avedro also manufactures CXL platforms compatible with transpeithelial (or epithelium-on) CXL protocol, the current label is intended only for standard epithelium-off CXL technique.
The immediate implications of this FDA approval are twofold. First, if a health insurance carrier decides to offer CXL coverage to beneficiaries diagnosed with keratoconus or ectasia, only epithelium-off treatments performed as per FDA label will likely be accepted. Second, an eye care practice that offers CXL may only receive liability insurance protections if it uses the FDA-approved apparatus. For a practice to employ other CXL devices and techniques, such as transepithelial CXL (TE-CXL), it would have to submit a separate Investigational New Drug Application to the FDA, as well as proof of Institutional Review Board oversights to be fully FDA compliant and receive liability protections.
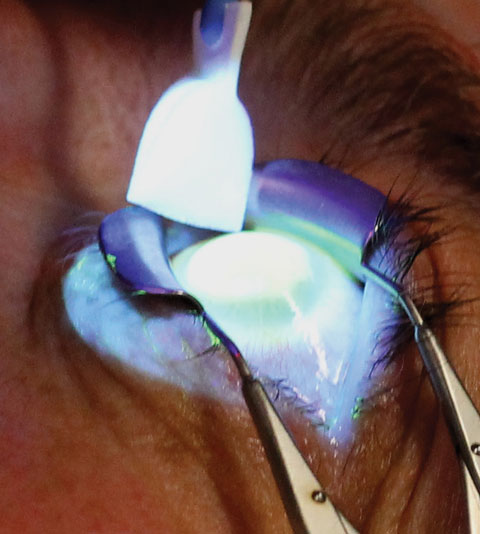 |
| Fig. 2. The clinician must tend to a patient’s eye during the UV emission stage of CXL. Photo: Marshall Ford, MD, Pacific Cataract and Laser Institute. |
CXL Protocol
Epithelium-off CXL, similar to a photorefractive keratectomy (PRK), is performed under topical anesthesia to ensure patient comfort. Through standard aseptic technique, epithelium is removed from the central 9mm to facilitate faster and more homogeneous riboflavin saturation within the corneal stroma. Photrexa viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) is then instilled on the cornea in two-minute intervals for 30 minutes.1
Since riboflavin serves dual functions as a photosensitizing agent to propagate CXL chemical reactions as well as a tissue protector by reducing UV transmittance beyond intended treatment depth, it is essential clinicians ensure full saturation within the corneal stroma prior to UV irradiation. This is done by checking for stromal and aqueous riboflavin staining in the slit lamp after the 30-minute riboflavin uptake phase (Figure 1).
A minimum of 400µm corneal thickness is required prior to the UV exposure phase, which is typically measured with an ultrasound pachymeter. Should pachymetry fail to show proper corneal thickness stipulated for treatment safety, hypotonic Photrexa riboflavin is administered every five to 10 seconds until the cornea is swollen to 400µm or greater.
Once clinicians have verified appropriate riboflavin saturation and pachymetry measurement, they can program the KXL UV device for 30 minutes of continuous emission (3mW/cm2) with a calibrated total energy dose of 5.4J/cm2.8 After UVA exposure is initiated, Photrexa viscous continues to be administered in two-minute intervals until the procedure is complete (Figure 2).
A bandage contact lens is maintained on the treated eye for three to five days or until epithelial closure. Although the exact ophthalmic medications may vary, patients are typically managed with topical antibiotic for one week and topical corticosteroid for two to three weeks.1
Candidates for CXL
The inclusion criteria set by the FDA for CXL includes a patient who: (1) is 14 years of age or older, (2) has a confirmed diagnosis of post-surgical ectasia or progressive KCN and (3) has a minimum stromal thickness of 400µm. However, disease severity and rate of progression tends to be worse in younger patients, stipulating a minimum age requirement of 14 excludes a large group of pediatric patients who may gain the most from CXL treatments.9 Therefore, many clinicians offer off-label treatment to younger patients, provided they can ascertain 400µm of stromal thickness.
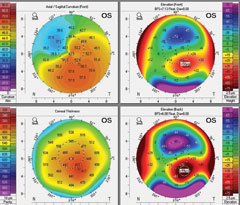 |
| Fig. 3. Above is a patient’s baseline tomography. Below, is the same patient’s tomography 14 months after CXL treatment. It shows mild apical flattening on the axial map (upper left) and reduced elevations maps (anterior elevation, upper right; posterior elevation, bottom right). Click images to enlarge. |
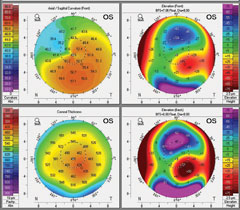 |
Given that post-surgical ectasia can occur at an older age than typically encountered for KCN, clinical consensus has not defined an age ceiling for these patients. Clinicians can recommend CXL in patients with post-surgical ectasia at any age based on documented progression and stromal thickness. For KCN, however, clinicians would be prudent to educate patients older than 40 years of age that CXL is a possible stabilization treatment option, but vigilantly monitor for evidence of progression prior to recommending CXL. Although we don’t have a general agreement on what magnitude of changes and time frame constitute progression, an expert panel recently recommended that at least two of the following three criteria need to be present to establish clinical progression: (1) progressive steepening in anterior corneal curvature, (2) progressive steepening of posterior corneal curvature or (3) progressive thinning when comparing pachymetric distribution profile from periphery to thinnest point.10
The same group of experts also asserted that waiting for documented progression prior to CXL may not be necessary in younger KCN patients since the likelihood of progression is almost certain, and the best CXL outcome can be obtained with early treatment.10
Recovery Process
The recovery of epithelium-off CXL is much like the recovery with any corneal epithelial delamination procedure, such as PRK. Although a bandage contact lens will enhance patient comfort, many may still experience ocular discomfort or pain until the epithelial defect closes, which usually occurs in three to five days. After wound closure, vision is expected to slowly change as epithelial remodeling and subsequent CXL effects manifest over time.
During the recovery phase, visual acuity was generally observed to worsen during the first month compared with preoperative presentation and slowly returns to baseline by the three-month follow up. Patients may experience some improvement in visual acuity between month three and six or thereafter until a trend of stabilization emerges as the new baseline. Research also shows a similar pattern of recovery after standard CXL with keratometry, pachymetry and corneal haze measurements—further steepening, thinning and reduction in tissue transparency were initially observed during the first month with trend reversal expected at around the third month, which may persist until stabilization (Figure 3). However, investigators found no clear associations between changes in corneal haze during the recovery phase and changes in other patient parameters such as best-corrected visual acuity (BCVA), maximum keratometry, mean keratometry and thinnest corneal point.1,9
Patients may misconstrue the worsening in these clinical parameters during the early postoperative period as CXL treatment failure; however, they need to be carefully monitored until at least month three or six when clinicians can assure a repeatable pattern prior to deciding if CXL treatment has taken effects.
Potential Benefits vs. Risks
Recent studies confirm the high level of efficacy with this standardized protocol—CXL with an epithelium removal technique—in halting keratoconus progression with a good safety profile. One meta-analysis on CXL demonstrated an average treatment success rate of 93.1%.11
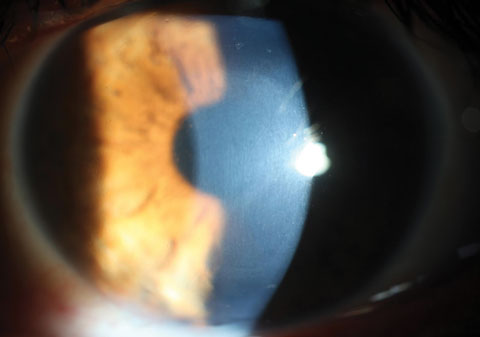 |
| Fig. 4. Using a slit lamp, clinicians can see CXL-associated corneal haze distributed within the treatment zone. Click image to enlarge. |
In addition to slowing or arresting keratoconus progression, numerous reports suggest improved topographic, refractive and aberrometric parameters after standard CXL.1,12,13 However, researchers have yet to discover specific preoperative patient variables that can reliably predict such postoperative improvements, so for now, patients should only be educated on corneal stabilization as the primary purpose of CXL rather than any potential refractive benefit.
Despite its good safety profile, CXL complications have been reported and should be part of our discussion with patients. Significant complications are rare and can include the common issues expected with any epithelial delamination process such as variable epithelial healing rate, eye pain, microbial keratitis and sterile infiltrates. Additionally, other CXL-related complications include corneal haze, persistent corneal edema/endothelial decompensation, unexplained loss of visual acuity and treatment failure. A closer look at these adverse events can help clinicians better manage patients should they arise.
Corneal haze. Unlike the rare event of PRK haze, CXL-associated corneal haze is common in the immediate post-CXL period (Figure 4); one study suggests it can be observed in up to 90% of patients after CXL, which significantly decreases on its own between three and 12 months.12 The same study also found no correlations between corneal haze and other postoperative parameters such as BCVA and keratometry. Therefore, CXL-associated haze likely represents a different cellular event compared with PRK haze. At the very least, it does not appear to share the same clinical concern as haze after PRK.
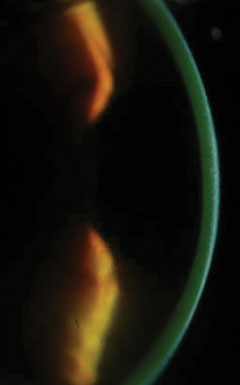
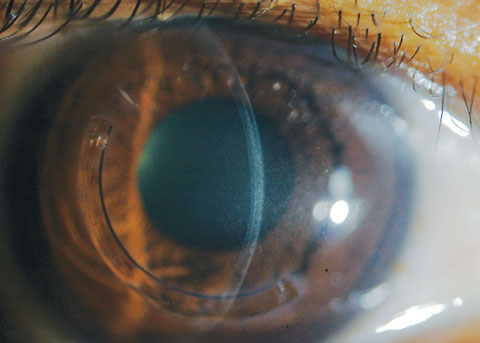 |
| Fig 5. A slit beam shows the demarcation lines, which are a potential indicator of CXL treatment depth in a patient who received off-label treatment of CXL and an Intacs corneal implant (AJL Ophthalmic). Click image to enlarge. |
Research suggests post-CXL haze, which occurs throughout the treatment zone in the anterior- and mid-stroma (as opposed to the reticular superficial scarring with PRK), is a result of increased density of extracellular matrix (ECM) with altered refractive indices in the treated tissue layers.14 This finding is often used as a histological indicator of CXL effects and its penetration depth—the posterior limit of the treatment zone is also referred to as demarcation line (Figure 5).1,14 Albeit uncommon, persistent and visually significant haze can occur, and some assert this may be more likely in patients with advanced KCN, highlighting yet another reason to attempt to diagnose the disease early in its course.15
Corneal endothelial decompensation. Part of the early development of CXL was to determine the endothelial cytotoxic threshold of UVA emissions. Investigators found the absence of cellular apoptosis if UV irradiance was below 0.35mW/cm2. The standard UVA CXL treatment protocol described in this article was determined to reach only 50% of this cytotoxic threshold, assuming a stromal thickness of at least 400µm and that sufficient riboflavin bioavailability is present within the stroma. With that said, studies have reported a few cases of non-resolving corneal edema requiring penetrating keratoplasty.15-17 Significant corneal thinning during the UV exposure phase may lead to such sequalae; thus, early CXL referral when ample corneal thickness is still present can help to prevent this rare complication.18
Loss of best spectacle-corrected visual acuity (BSCVA). Overwhelmingly, the majority of eyes either maintain the same or slightly improved BSCVA compared with baseline profile; however, studies reveal reduced BSCVA in a small group of eyes with no apparent link to other clinical parameters to explain such episodes.1,19 Investigators estimate the risk for losing two lines or more in Snellen acuity after CXL treatment is between 1.4% and 3.5%.1,19 Fortunately, this finding does not correlate with BCVA achieved with contact lenses when patients are ready for contact lens fitting after CXL.
Treatment failure or regression. Treatment failure is rare, as research shows approximately 2% to 8% of patients have continued worsening of ectatic disease after CXL.1,12 However, such diagnosis may take time to formulate, given the expected keratometric steepening and corneal thinning during the first one to three months immediately after CXL. Nonetheless, researchers have observed long-term treatment regression from many clinical trials, with more reporting of this complication after TE-CXL and in the pediatric patient group (18 or younger).20-22
The Benefits of Combined ProceduresBy A. John Kanellopoulos, MD Internationally, CXL is often combined with customized partial surface ablations, such as LASIK, to help patients become spectacle- or contact lens-independent. Known as the “Athens Protocol,” the technique—extensively documented, even with 10 years of follow up—practiced globally as an option in lieu of CXL alone, with higher visual rehabilitation benefits. Now that both CXL and topography-guided ablations are approved in the United States, the actual clinical prevalence of the technique will soon be determined.1-5 Besides treating ectasia, researchers can perform smaller amounts of CXL, which is proven to aid as a biomechanical modulator (enhancer) in routine LASIK procedures in both myopia and hyperopia.1-5
|
Epithelium-On or -Off?
Despite its proven efficacy and minimal invasiveness, investigators have raised clinical concerns over the epithelial removal technique, primarily due to its association with adverse events. While research shows fewer associated complications when the epithelium is left minimally disrupted, numerous reports also demonstrated lowered CXL efficacies with TE-CXL, which may necessitate more frequent retreatment with CXL across a patient’s life time.20-22 This may be because an intact epithelial barrier prevents riboflavin from gaining entry into stromal tissue as well as slow down stromal oxygen diffusion.
In addition, a patient noncompliant with follow up and with higher risk factors for progression may have more cumulative risks for progression between each CXL treatment. Given the primary purpose of CXL is stabilizing against progression, many investigators still favor the standard epithelium-off CXL technique due to its higher yield in treatment efficacy. Still, TE-CXL not only has fewer reports of associated adverse events, patients also experience a quicker recovery course; hence, continual research efforts will likely bring about enhanced treatment effects in TE-CXL in the future.11,22
Contact Lenses After CXL
Despite findings of improved topographic, refractive and aberrometric characteristics post-CXL, most patients will still require contact lenses to achieve best visual outcome. With advancements in contact lens technologies, we now have myriad lens options to offer to our patients to accommodate their different disease severities and visual needs (Figure 6).
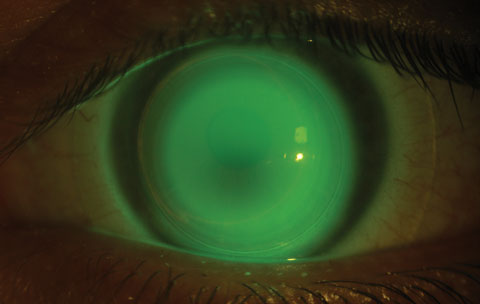 |
| Fig. 6. This patient was successfully fit in a hybrid contact lens after standard epithelium-off CXL. Click image to enlarge. |
Patients’ K values will flatten by two diopters, on average, in the first year after standard epithelium-off CXL, with most of that effect occurring in the first six months. Habitual lens wearers may resume lens wear as early as four weeks. In these cases, clinicians should instruct them to bring their habitual lenses during their one-month exam to ensure a reasonable fitting relationship with adequate visual function. If the previous lens modality provides a reasonable fitting relationship, adequate vision and good comfort, patients can continue wearing them throughout the initial six to 12 months to reduce the cost of frequent lens replacement within the first year after CXL. If needed, clinicians can refit a patient into a new lens as early as six months, as the majority of the topographical changes have already occurred and subsequent changes are expected to exert less impact on the patient’s corneal elevation profile.
For non-contact lens wearers or if the previous lens modality exhibits a poor lens fitting relationship at the one-month visit, clinicians may fit patients earlier to provide necessary visual abilities to perform daily tasks. As long as patients understand the possibility of frequent lens replacements during the first year, clinicians can consider a refit or a new fit as early as four to six weeks after standard epithelium-off CXL.
Despite some evidence showing reduced treatment efficacy with TE-CXL protocols, patients do experience quicker visual recovery courses after such treatment.11,20-22 Therefore, TE-CXL patients can often resume lens wear or be refit in to a new lens at one to two weeks after TE-CXL.
Take Home Message
CXL is an enormous change in the treatment algorithm of KCN and other keratectasias—it is now more crucial than ever to be mindful of early disease detection. Previously, clinicians had to tell patients their conditions would naturally worsen over time and that keratplasty might not be avoidable. But today, CXL can help prevent these limitations, allowing patients to function at their absolute best.
Identifying these patients as early in the disease process as possible will allow them the option of earlier treatment to avoid the many barriers imposed by their ocular disorder across different stages of their lifespans. With the suspected higher disease prevalence and the positive impact we can make with early detection, proper keratoconic screening and timely referral for CXL will soon become the standard of care for our profession.4-5
Dr. Chang is director of cornea specialty lenses at Wills Eye Hospital–Cornea Service and director of clinical services at TLC Vision. Also, he is an advisory board member for the International Keratoconus Academy, the Gas Permeable Lens Institute and the Optometric Cornea, Cataract and Refractive Society.
Dr. Bronner is an attending optometrist at Pacific Cataract and Laser Institute in Boise, WA.
|
1. Chang CY, Hersh PS. Corneal collagen cross-linking: a review of 1-year outcomes. Eye Contact Lens. 2014;40(6):345-52. 2. Hashemi H, Seyedian MA, Miraftab M, et al. Corneal collagen cross-linking with riboflavin and ultraviolet a irradiation for keratoconus: long-term results. Ophthalmology. 2013;120(8):1515-20. 3. O’Brart DP, Kwong TQ, Patel P, et al. Long-term follow-up of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linking to halt the progression of keratoconus. Br J Ophthalmol. 2013;97(4):433-7. 4. Godefrooij DA, de Wit GA, Uiterwaal CS, et al. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017 March;175:169-72. 5. McGhee CN, Kim BZ, Wilson PJ. Contemporary treatment paradigms in keratoconus. Cornea. 2015;34(Suppl 10):16-23 6. Duncan JK, Belin MW, Borgstrom M. Assessing progression of keratoconus: novel tomographic determinants. Eye and Vision. 2016;3:6. 7. Kuo IC, Broman A, Pirouzmanesh A, Melia M. Is there an association between diabetes and keratoconus? Ophthalmology. 2006;113(2):184-90. 8. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-7. 9. Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28(11):763-7. 10. Gomes JA, Tan D, Rapuano CJ, et al; Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-69. 11. Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye (Lond). 2015;29(1):15-29. 12. Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37(1):149-60. 13. Vinciguerra P, Albè E, Trazza S, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116(3):369-78. 14. Moramarco A, Iovieno A, Sartori A, Fontana L. Corneal stromal demarcation line after accelerated crosslinking using continuous and pulsed light. J Cataract Refract Surg. 2015;41(11):2546-51. 15. Sharma A, Nottage JM, Mirchia K, et al. Persistent corneal edema after collagen cross-linking for keratoconus. Am J Ophthalmol. 2012;154(6):922-6. 16. Gokhale NS. Corneal endothelial damage after collagen cross-linking treatment. Cornea. 2011;30(12):1495-8. 17. Bagga B, Pahuja S, Murthy S, Sangwan VS. Endothelial failure after collagen cross-linking with riboflavin and UV-A: case report with literature review. Cornea. 2012;31(10):1197-200. 18. Sharma A, Mohan K, Niranakari VS. Endothelial failure after collagen cross-linking with riboflavin and UV-A: case report with literature review. Cornea. 2013;32(7):e180-1. 19. Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009;25(9):S824-8. 20. Caporossi A, Mazzotta C, Paradiso AL, et al. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39(8):1157-63. 21. Soeters N, Wisse RP, Godefrooij DA, et al. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 2015;159(5):821-8.e3. 22. Kocak I, Aydin A, Kaya F, Koc H. Comparison of transepithelial corneal collagen crosslinking with epithelium-off crosslinking in progressive keratoconus. J Fr Ophtalmol. 2014;37(5):371-6. |

