A 77-year-old white male presented for a glaucoma follow-up visit and complained of a slowed ability to adjust from light to dark. He reported no decrease in vision.
The patients ocular history is positive for primary open-angle glaucoma in both eyes, for which he took Xalatan (latanoprost, Pfizer) 1 drop hs O.U. Ocular history also included a +2 to +3 nuclear sclerotic cataract in the right eye, and pseudophakia and vitreous floaters in the left eye.
The patients systemic history was significant for diabetes, chronic airway obstruction, prostate cancer and hypertension. He used the following medications: albuterol 17g and Aerobid (flunisolide, Forest) 7g inhalers for the chronic airway obstruction, glyburide 5mg for his diabetes, and Zoladex (goserelin acetate, AstraZeneca) 10.8mg injectable implant and Nilandron (nilutamide, Aventis) 150mg for the prostate cancer.
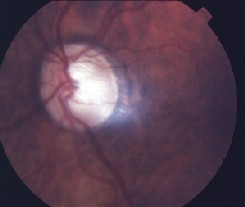
A dilated fundus examination revealed vitreous floaters in our patient O.S.
Diagnostic Data
Uncorrected acuity was 20/40 O.U. Pinhole acuity showed no improvement in either eye. Pupils were equal, round and reactive with no afferent pupillary defect. Color testing with Ishihara color plates resulted in 24/24 for each eye. Extraocular muscles were unremarkable. Confrontational visual fields were normal.
Biomicroscopy revealed a +2 to +3 nuclear sclerotic cataract O.D. and a clear posterior chamber intraocular lens O.S. The remainder of the slit lamp exam was unremarkable O.U.
Intraocular pressure measured 17mm Hg O.D. and 18mm Hg O.S. Amsler grid testing was unremarkable. Photostress recovery time was 4 minutes, 38 seconds O.D. and 4 minutes, 45 seconds O.S.
A dilated fundus examination revealed vitreous floaters O.S. The cup-to-disc ratio was 0.75/0.75 O.D. and 0.85/0.85 O.S. There was no posterior pole pathology in either eye.
Diagnosis
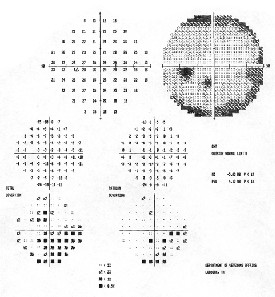
Visual fields were normal in our patient, O.D. and O.S.
I diagnosed this patient with poor photostress recovery time as a side effect of the nilutamide.
Treatment and Follow-up Photostress Testing The photostress test, as used on this patient, can help clinicians differentiate between macular and optic nerve disease. 1. Alexander L. Macular Photostress Recovery Test. In: Alexander L. Primary Care Of The Posterior Segment. San Mateo: Appleton and Lange, 1989:9-10.
I instructed the patient to continue with the latanoprost and notified his urologist about the side effects he experienced from the cancer treatment. I scheduled the patient for a follow-up visit in two months.
At follow-up, the patient reported that his urologist discontinued the nilutamide the same day we reported the side effect. The patient said that he was now taking Casodex (bicalutamide, Astra-Zeneca) 50mg once daily by mouth. The patient said his symptoms improved after two weeks.
His ocular health remained unchanged from the previous visit. Visual acuity improved to 20/30 O.D. and O.S. A repeat photostress test revealed improved dark adaptation, with a recovery time of 53 seconds O.D. and 51 seconds O.S.
Discussion
Nilutamide is a nonsteroidal anti-androgen agent used in the treatment of prostate cancer. It prevents the normal androgenic response by interacting with the androgen receptors. Prostate cancer is sensitive to the reduction of testosterone. Anonsteroidal antiandrogen agent reduces testosterone to help reduce the prostate cancer.1,2
Clinical trials of nilutamide have shown that 13% to 57% of patients reported a delay in dark adaptation when going from light to dark areas.1 In one Italian study, a third of patients reported im-paired dark adaptation.3 Other visual side effects included slight nausea (26.9% of patients) and alcohol intolerance (19.2%).
The test involves bleaching the retinal pigments by exposing the retina to bright light for an extended period, then measuring visual recovery time. Visual recovery time is the time it takes for a patients photoreceptors to recover from the light exposure. Recovery time is dependent on the ability of the photoreceptors to complete the photochemical cycle required to resynthesize visual pigments.1
This monocular test is based on the patients best-corrected visual acuity in the eye being tested. To perform this test:
1. Have the patient adapt to a darkened room for one minute.
2. Hold a transilluminator or indirect ophthalmoscope approximately 3cm in front of the eye for 10 seconds. Ask the patient to focus on the light.
3. Have the patient read the letters one line above his or her best-corrected acuity. The time between illumination and the patients ability to read this line is the photostress recovery time.
A photostress recovery time of more than 50 seconds is considered abnormal.1 A reduction in visual acuity with a delayed photostress recovery time indicates a probable maculopathy, while a reduction in visual acuity with a normal photostress test indicates a probable problem with the optic nerve.B.E.L.
Approximately 65% of patients in one study reported a delay in dark adaptation after exposure to a bright source of illumination.4 Types of illumination included bright light, sun and television. The patients in this study exhibited an average photostress recovery time of 9 minutes. When these patients were switched to flutamide, another antiandrogen, the photostress recovery test time decreased significantly, and visual side effects resolved.4
Anecdotally, I examined two other patients who took nilutamide. Both reported delayed dark adaptation time. They also reported white-colored objects appeared pinkish in color. (This patient did not report this symptom.) Both patients had normal color testing with Ishihara color plates.
Warn patients who take nilutamide about the potential visual side effects. If a patient who has prostate cancer takes nilutamide and experiences delayed dark adaptation, dyschromatopsia or delayed light adaptation, notify the prescribing physician. The practitioner might be able to provide an alternative agent such as Casodex or Eulexin (flutamide, Schering). Neither of these medications has been associated with delayed dark adaptation4,5. Visual side effects from nilutamide tend to improve dramatically after a change in medication.
This case demonstrates the importance of knowing our patients systemic health history and keeping up-to-date on any changes in health status or medication. Staying aware of these changes, and their potential effects on the visual system, will enable us to help improve our patients overall health and treatment.
Dr. Bryan Lallathin practices at the Department of Veterans Affairs Outpatient Clinic in Lubbock, Texas.
1. Aventis Pharmaceuticals, Nilutamid Prescribing Information as of March, 2004. http://www.aventispharma-us.com/PIs/nilandron_TXT.html.
2. Tyrrell CJ. Casodex: a pure non-steroidal anti-androgen used as monotherapy in advanced prostate cancer. Prostate Suppl 1992;4:97-104.
3. Decensi AU, Boccardo F, Guarneri D, et al. Monotherapy with nilutamide, a pure nonsteroidal antiandrogen, in untreated patients with metastatic carcinoma of the prostate. The Italian Prostatic Cancer Project. J Urol. 1991 Aug;146(2):377-81.
4. Harnois C, Malenfant M, Dupont A, Labrie F. Ocular toxicity of Anandron in patients treated for prostatic cancer. Br J Ophthalmology 1986 Jun;70(6):471-3.
5. Mahler C, Verhelst J, Denis L. Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer. Clin Pharmacokimnet. 1998 May;34(5):405-17.
6. Alexander L. Macular Photostress Recovery Test. In: Alexander L. Primary Care Of The Posterior Segment. San Mateo: Appleton and Lange, 1989:9-10.

