 |
A 54-year-old Caucasian male was referred from his primary care physician for pain, redness and photophobia in the left eye for one month, which had been refractory to palliative therapy with artificial tears and warm compresses. Additional history questions revealed transient symptoms of vertigo, right arm weakness and left facial numbness, also for one month. He denied pain, hearing or weight loss, and his gait was unaffected. Past ocular, social and family histories were unremarkable.
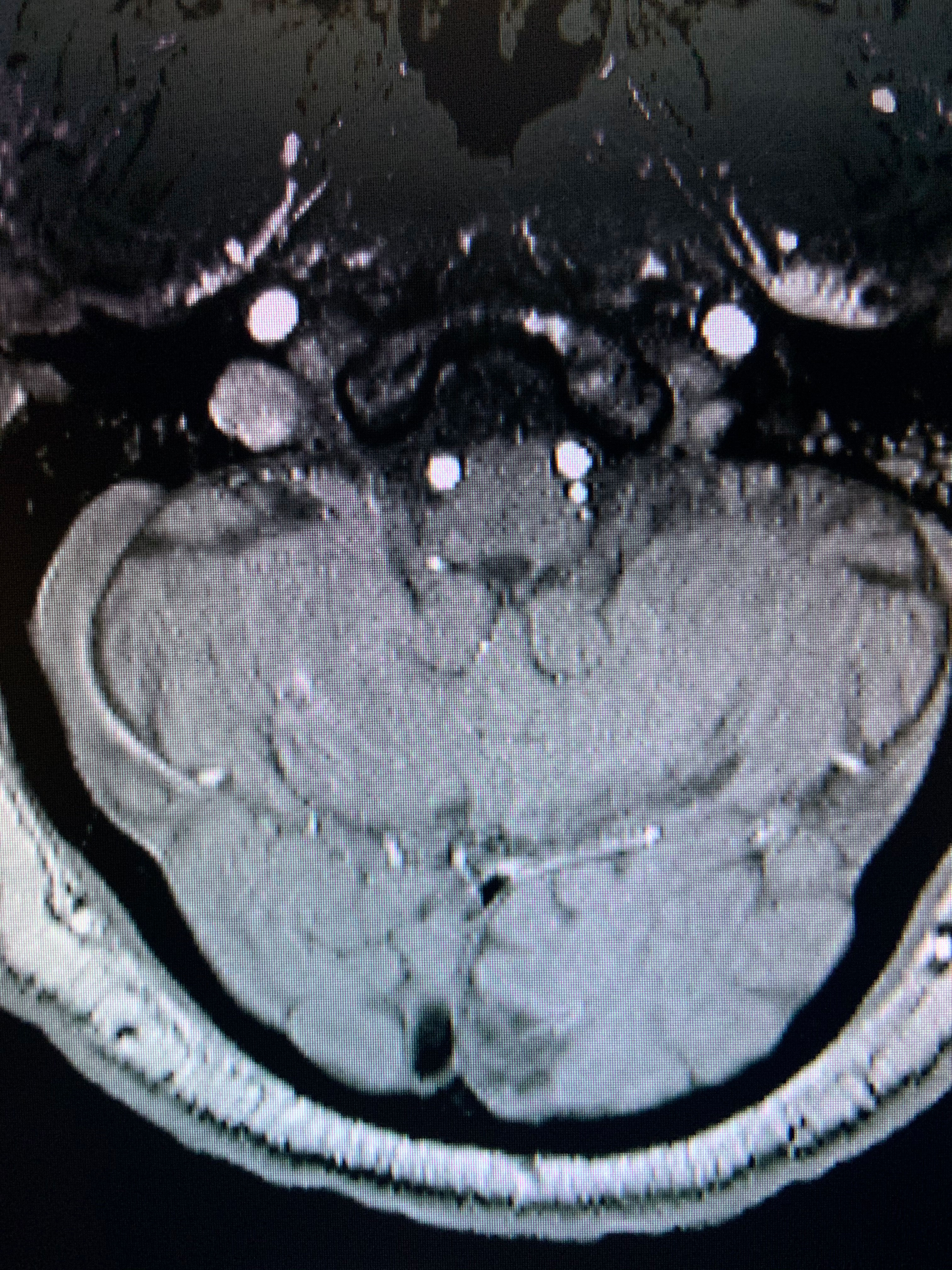 |
| Ischemic stroke within the left occipital cortex can lead to a right hemianopia. Click image to enlarge. |
Evaluation and Diagnosis
Best-corrected visual acuity measured 20/20 OD and 20/50 OS. Confrontation field testing revealed a questionable right hemianopia. Subsequently, a formal HVF 30-2 revealed an incongruous right hemianopia. Extraocular muscle testing revealed a left abduction deficit that was neutralized with six prism diopters (base out).
Sensory testing of the face revealed hypoesthesia confined to the left cheek. Saccades were normal in magnitude and velocity in right gaze but equally diminished in left gaze. Examination of the left eye showed diffuse conjunctival hyperemia without discharge and grade 4 punctate epithelial erosions without infiltrates or dendrites. The lens showed mild nuclear sclerotic changes in each eye.
Reduced sensation of the left cheek is most indicative of maxillary division (V2) involvement of the trigeminal nerve—cranial nerve (CN) V. This prompted us to evaluate the ophthalmic division (V1) of CN V. Reduced facial and corneal sensation indicated CN V involvement, whereas reduced basal tear secretion identified motor involvement of the facial nerve (CN VII).
The symptoms and signs of right arm weakness, vertigo, right hemianopia, left abduction deficit, reduced sensory innervation of V1 and V2 and lacrimal gland dysfunction led us to a diagnosis of presumed polyneuropathy. Subsequently, an emergent referral was made to the emergency department for combined MRI/MRA with special attention to the brainstem. MRI was ordered to assess for soft tissue changes associated with stroke or tumor, while MRA allows for direct visualization of blood flow. A combined MRI/MRA is most useful when evaluating for potential vascular pathology (i.e., stroke, aneurysm or arteriovenous malformation).
Results of neuroimaging indicated (non-acute) brainstem infarction involving the left lower lateral pons including the sixth, seventh and eighth nerve nuclei, the posterior limb of the left internal capsule and the left occipital cortex. Additionally, there were moderate white matter lesions dispersed throughout both hemispheres due to chronic microvascular disease.
Subsequently, the patient was scheduled for consultations in the cardiology and neurology departments. His medications for diabetes, hypertension and cholesterol were changed and he was placed on a blood thinner. He was eventually discharged and prescribed physical therapy. Additionally, we treated the keratopathy with a combination of Prokera (amniotic membrane, Bio-Tissue), punctal occlusion and Restasis (cyclosporine A 0.05%, Allergan) BID. Over the next several months, the left abduction deficit resolved, his right arm regained strength and there was a dramatic improvement in keratopathy with his best-corrected vision improving to 20/20 OS.
Discussion
Ischemic infarction accounts for 80% to 90% of all strokes, with the remaining 10% to 20% due to hemorrhagic stroke.1,2 Of the ischemic strokes, 25% involve the posterior circulation.1,2 Of these, 60% and 40% occur in the brainstem and cerebellum, respectively.1,2 Brainstem infarctions are classified according to three main groups representing the site of infarction: mesencephalon, pons and medulla. Each of the three anatomic locations are further subdivided into anteromedial, anterolateral, lateral and posterior segments.3
In our patient, ischemic infarction occurred within the left lower lateral pons, left posterior limb of the internal capsule and the left occipital cortex.
Clinicians should conduct a short but efficient neuro exam in the office to assess for multiple cranial nerve involvement. Assess the sensory component of the ophthalmic division of CN V by gently touching a small bundle of cotton swabs to each cornea to compare sensation and evaluate the corneal reflex.
Similarly, use a pinprick to test sensation of both the maxillary and mandibular divisions. A physical evaluation of the motor functions of CN VII includes asking the patient to smile, close their eyes and wrinkle their forehead.
Test limb strength, gait and balance by simply applying force to the arms and legs while comparing their individualized resistance. Ask the patient to attempt to walk in a straight line and carefully instruct them to lift one leg off the ground while standing in position. Keep a low threshold when distinguishing normal from abnormal and practice performing these tests on unaffected patients.
The following describes the association between the neuroanatomy involved and the symptoms our patient presented with:4
- left internal capsule (subcortex): weakness of the right arm and hand
- left occipital cortex: right hemianopia
- CN VI: left abduction deficit
- V1, V2 and spinal trigeminal nucleus: loss of pain sensation from the cornea and face, respectively
- facial nerve: reduced basal tear secretion
- vestibular nuclei: vertigo
The internal capsule allows for communication between the cerebral cortex and the brain stem regarding motor and sensory activity from the arm, leg, trunk and face. The internal capsule consists of three parts: genu, anterior limb and posterior limb.
In our patient, ischemic infarction of the left posterior limb of the internal capsule resulted in contralateral hemiparesis and reduced sensation in the right arm and hand.4
Confirming a Stroke
Signs and symptoms of internal capsular stroke may include a combination of the following: weakness of the face, arm or leg; upper motor neuron signs including hyperreflexia, Babinski sign, clonus and spasticity; and mixed sensorimotor, which involves motor fibers from the arm, trunk and legs and sensory fibers that leads to contralateral weakness and contralateral sensory loss.4 Lastly, the occipital cortex houses the visual cortex—the visual processing center. In our patient, ischemic infarction within the left occipital cortex resulted in an incongruous right hemianopia.
Ocular complications included left abduction deficit, severe keratopathy and incongruous hemianopia. An abduction deficit could be managed with observation, vision therapy or prism. In this case we felt the keratopathy was severe enough to warrant immediate application of an amniotic membrane such as Prokera, a cryopreserved amniotic membrane that contains nerve growth factor, which was found to accelerate healing and re-epithelialization of the ocular surface as well as stimulate corneal nerve regeneration.5
In addition, we prescribed Restasis to reduce secondary inflammatory from neurotrophic disease. We performed punctal occlusion, after reducing inflammation, in order to increase the tear lake. Because the visual field defect was not causing a noticeable problem in our patient, we conservatively managed this with observation and periodic visual field testing. However, if the defect were robust enough to cause awareness of the field loss, we could have prescribed yoked prism with or without occupational therapy.
Interestingly enough, our patient’s chief complaint was a red, painful eye, albeit he presented with more concerning clinical findings that led to the diagnosis of brain stem infarct. Because dry eye complaints are so frequent, it is prudent to consider alternative diagnoses if there is considerable inter-ocular asymmetry, as was observed in our patient.
The combination of ocular and neurological signs and symptoms were clues to investigate for a neurologic process. A good understanding of anatomy and pathophysiology allowed us to better understand the mechanism underlying the presenting symptoms, which then led to additional CN testing and appropriate neuroimaging studies.
1. Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521-6. 2. Bogousslavsky J, Regli F, Maeder P, et al. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance imaging and magnetic resonance angiography. Neurology.1993;43(8):1528–33. 3. Burger KM, Tuhrim S, Naidich TP. Brainstem vascular stroke anatomy. Neuroimag Clin N Am. 2005;15(2):297–324. 4. Campbell WW. DeJong’s The Neurologic Examination. 7th ed. Philadelphia, PA: Lippincott, Williams & Wilkins. 2012:338. 5. John T, Tighe S, Sheha H, et al. Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease. J Ophthalmol. 2017; 6404918. |

