
A relative afferent pupillary defect (RAPD) can be a worrisome exam finding, so an understanding of its possible etiologies is crucial. Most often, patients have an ocular or medical history that might explain its presence. For example, a patient with a history of total retinal detachment in one eye would manifest a relative afferent pupillary defect. However, some patients present with sudden, gradual or no known vision loss and manifest an RAPD for the first time in your office.
Now what? Is this an emergency? What do you do now? For instance, an RAPD in the eye of an 80 year old patient with sudden vision loss may be secondary to giant cell arteritis.
Here are some clinical examples of an APD, its causes and the proper steps to take when managing these patients.
Pathway Anatomy
The pupillary light reflex begins in the retina.1-3 There are retinal ganglion cells that serve the pupillary light reflex only, and about 53% of these fibers decussate at the optic chiasm.4 These represent the nasal fibers. The temporal fibers remain ipsilateral.
Both fibers follow the optic tract and separate from it prior to the lateral geniculate nucleus. The fibers then synapse with the pretectal nuclei at the level of the midbrain. From here, light fibers from the pretectal nuclei innervate both Edinger-Westphal nuclei.1-3 This is the origin of the consensual light reflex and the reason that an optic nerve or an optic tract lesion does not result in anisocoria. Efferent fibers from the Edinger-Westphal nuclei, the autonomic subnucleus of the third cranial nerve, result in pupillomotor and ciliary muscle innervation.1 These preganglionic fibers follow the third nerve into the orbit and synapse in the ciliary ganglion. Postganglionic fibers follow the short posterior ciliary nerves and run in the suprachoroidal space to innervate the pupillary sphincter.1-3
The pupillary light reflex is the summation of the entire light pathway. So, damage anywhere along the pathway can reduce conduction of the pupils response to light.5 You can detect asymmetric damage between the eyes by comparing direct and relative responses between pupils.
Additionally, the pupillary light reflex summates the entire visual field. So, pupil testing can assess the amount of relative functional visual field between eyes.5
Most commonly, the eye that manifests the RAPD has ipsilateral damage prior to the chiasm. For example, an RAPD may be due to an ipsilateral optic nerve glioma. In contrast is the unique example of an optic tract lesion. In this case the RAPD is present in the eye in which the temporal visual field has been damaged. This supports the existence of greater decussating nasal fibers at the optic chiasm. Therefore, the RAPD will appear in the eye opposite the optic tract lesion.4,6
Possible Diagnoses
Some articles suggest that an RAPD can occur as a result of the light scatter caused by a very dense asymmetric cataract. Clinically, however, a dense cataract should never be used to explain the presence of an RAPD.7
The presence of an RAPD most often indicates relative optic nerve disease.8 An amblyopic eye may present with a mild RAPD, but you must first rule out optic nerve pathology.
Also, an RAPD can be caused by significant retinal pathology. For instance, a large macular scar, a significant retinal detachment and a central retinal artery or ischemic central retinal vein occlusion can result in an afferent pupillary defect.1-3
I suggest taking a stepwise approach when a patient presents with an RAPD. Specifically:
Ask if the patient is aware of changes in vision. If so, are these changes acute or gradual? An acute optic neuropathy or retinal detachment would be associated with sudden vision loss, while gradual vision loss might support the existence of a compressive lesion.
Elicit significant ocular and medical history. Does the patient have a history of vascular disease, cancer, auto-immune disease, recent infections or trauma?
Carefully examine the patient, and pay special attention to the retina and optic nerve. Optic nerve findings can be subtle or non-existent. For instance, a relatively normal appearing nerve might be present in a retrobulbar optic neuritis. That is why color vision testing and perimetry are so important to the evaluation.
The need to refer for further testing depends on the case. If the patients history and retinal examination do not offer an obvious explanation for the APD, one must assume that a condition affecting the optic nerve or optic tract is causing it. An immediate visual field and color vision test should be performed.
Depending on the condition, the patient may need to be referred for emergent neuro-imaging and laboratory testing. For example, an individual who presents with an RAPD as a result of swollen optic nerve and a history of leukemia, would require emergent imaging and radiation treatment if the swollen nerve represented a leukemic infiltrative optic neuropathy.
The following cases illustrate the importance of differential diagnosis and exam findings in establishing the cause of an RAPD.
Case 1: Gradual Vision Loss
A 59-year-old white male presented complaining of gradually decreasing vision in his right eye. His ocular history was remarkable for blunt ocular trauma in the same eye 10 years ago. His medical history was positive for hypertension, hepatitis C and migraine headaches. His current medications are hydrochlorothiazide and Topamax (topiramate, Ortho-McNeil-Janssen Pharmaceuticals).
The patients entering and best-corrected visual acuity was 20/200 O.D. and 20/20 O.S. His pupils were equal, round and slightly less reactive to light O.D., with an RAPD O.D. Confrontation visual fields were superiorly constricted O.D. and full to finger-counting O.S. He had full color plates O.S., but was unable to see the test plate O.D. Slit lamp examination of the anterior segment revealed sectoral iris atrophy O.D. and a small, peripheral corneal scar O.S.
His intraocular pressure (IOP) measured 29mm Hg O.D. and 13mm Hg O.S. Gonioscopy revealed wide open angles O.U. with grade 3+ pigment O.D. and trace pigment O.S.
A dilated fundus exam showed a cup-to-disc ratio of 0.99 x 0.99 O.D. and 0.3 x 0.3 O.S.The retinal nerve fiber layer was largely absent O.D. and normal O.S. (figures 1 and 2). The inferior neuroretinal rim appears obliterated Each macula and peripheral retina were unremarkable.
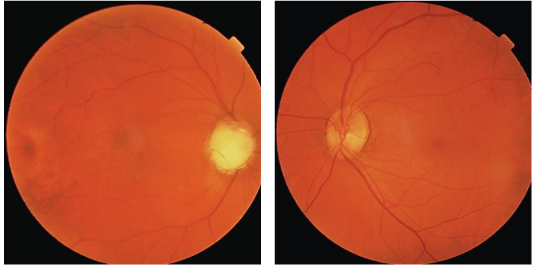
1, 2. A dilated fundus exam in this patient with traumatic glaucoma showed that the retinal nerve fiber layer was largely absent O.D. (left) and normal O.S. The inferior neuroretinal rim appears obliterated.
A Humphrey 24-2 SITA Standard confirmed a superior field defect involving fixation in the right eye and a full visual field in the left (figures 3 and 4).
Diagnosis. The patient has traumatic glaucoma O.D.
Treatment and follow-up. We educated the patient about his condition and started him on timolol 0.5 % b.i.d. O.D. and Travatan Z (travoprost, Alcon) q.h.s. O.D. We instructed him to return for follow-up in two weeks.
We set a target IOP circa 10mm Hg. At his follow-up, his IOP was 11mm Hg O.D. and 9mm Hg O.S.
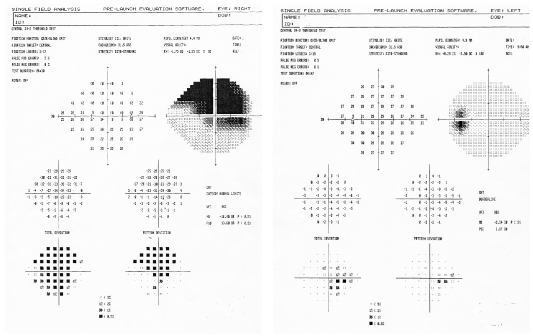
3, 4. A Humphrey 24-2 SITA Standard confirmed a superior field defect O.D. involving fixation and a full visual field O.S.
Discussion. Certainly glaucomatous optic nerve damage O.D. was responsible for the APD. Given the high IOP, glaucoma was by far the most likely cause.
Although a history of blunt trauma accounts for the unilateral presentation in this case, such asymmetry is uncommon in glaucoma. Without a history of trauma, a compressive lesion might have been possible with incidentally elevated IOP. Note that the APD would have been present even with near normal visual acuity, given the difference in cupping.
If the patients IOP was low and there was a history of trauma, you might consider glaucoma that has burned out, but you would first need to rule out non-glaucomatous optic neuropathy by imaging the patient.
Finally, our patient was using Topomax, an anti-seizure/migraine medication that has a history of causing acute glaucoma via posterior pupillary block.9 But, such a presentation would have been acute, with higher IOP, associated pain and choroidal detachment.9 A cupped-out nerve would be unlikely.
Case 2: Amaurosis Fugax
A 53-year-old black male returned to the clinic for a one-week follow-up secondary to episodes of intermittent graying-out of vision O.D. lasting about 20 minutes each. There were no associated symptoms. His initial eye examination had been clinically unremarkable.
His medical history was significant for poorly controlled hypertension and hypercholesterolemia, for which he took atenolol and simvastatin, respectively. He denied weakness, numbness, headache or speech difficulty.
Given his symptoms, however, we sent the patient for an emergent complete blood count (CBC) with differential, prothrombin time (PT) and partial thromboplastin time (PTT) tests, platelets, carotid duplex and echocardiogram; all were normal.
As a precaution, we started him on 325mg of aspirin and told him to return to clinic in one week for follow up. We also told him to report immediately to the emergency room if he noticed any non-recovering vision loss or peripheral weakness or numbness.
At follow-up, the patient reported that the vision in his right eye worsened four days following his last eye examination, and that he could only see light and gross figures at that time. He denied any pain and peripheral weakness or numbness. The patient claimed that he did not have a chance to report for evaluation after the vision loss.
His entering visual acuity without correction was counting fingers at one foot, with no improvement on pinhole acuity O.D. and 20/30+2 O.S. At his examination one week earlier, his acuity had been 20/25 O.D. and 20/30 O.S.
His pupils were equal, round and reactive to light, with a relative afferent pupillary defect in the right eye. The patient was unable to respond to confrontation visual fields in the right eye and was full to finger counting in his left eye. His IOP measured 14mm Hg O.U. The slit lamp examination was unremarkable.
A dilated fundus examination revealed an edematous optic nerve with white edematous and ischemic areas in all four quadrants of the right eye (figure 5). The left eye was unremarkable.
Diagnosis. The patient has a central retinal artery occlusion of the right eye which was preceded by episodes of amaurosis fugax.
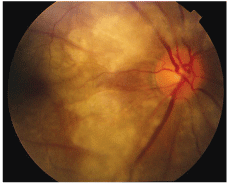
5. A dilated fundus examination in this patient with central retinal artery occlusion revealed an edematous optic nerve O.D. with white edematous and ischemic areas in all four quadrants.
Treatment and follow-up. No treatment was indicated because the occlusion occurred more than 24 hours before the patient presented. The retina has already been destroyed by ischemia.10 We consulted a retinal specialist, who did not recommend fluorescein angiography.
We educated the patient about his condition and advised him that his vision was unlikely to improve. We also referred him back to his primary-care physician for additional evaluation and gave him the same precautions as at the end of the prior examination.
The patient was scheduled for follow-up in the eye clinic in one month. At follow-up, there was no improvement in vision.
Discussion. Amaurosis fugax is an ophthalmic and medical emergency. Most commonly, it is caused by emboli thrown from the carotid artery or the heart; this eventually causes occlusion of the retinal arteriole system and/or an occlusive event anywhere else in the body.
Patients with amaurosis fugax usually have a history of hypertension, diabetes or hypercholesterolemia. As a result, the patient is in danger of cerebral vascular accident (CVA) in addition to the ocular sequelae.
So, patients must be referred to the emergency room to rule out emboli actively being thrown from the carotid artery, cardiac valves or aortic arch. If a patient with these symptoms is 56 or older, one must consider giant cell arteritis (GCA).11 Other conditions, such as antiphospholipid antibody syndrome and systemic lupus erythematous, may also result in arteriole occlusion. The patient was not worked up for these conditions.
A central artery occlusion of less than 24 hours might be aided by intervention, including lowering IOP, performing ocular massage and attempting vasodilation by increasing carbon dioxide levels in the blood (this is accomplished by having the patient breathe into a paper bag). Clot-busting thrombolytic agents have shown some success.12,13
However, it is difficult to differentiate between successful treatment and spontaneous resolution.
Whether your patient presents with an afferent pupillary defect caused by an identifiable central retinal artery occlusion or only with symptoms of amaurosis fugax, he or she requires an urgent medical work-up to avoid potential CVA and/or sudden death.
Case 3: My Vision is Blurry
A 52-year-old white male presented with blurry vision in his right eye upon awakening. He denied any history of trauma, eye pain or amaurosis fugax. His medical history is positive for hypertension, for which he takes lisinopril.
His entering acuity and pinhole vision were 20/100 O.D. and 20/20 O.S. His extraocular motilities were full, with no pain on eye movements. Confrontation visual fields were constricted inferiorly in the right eye and full to finger-counting in the left. His pupils were equal, round and reactive to light, with a mild RAPD O.D. The patient was able to see four out of 14 color plates O.D. and all plates O.S. The slit lamp examination revealed blepharitis O.U. IOP measured 17mm Hg O.D. and 15mm Hg O.S.
A dilated fundus exam illustrated a hyperemic, edematous optic nerve in the right eye, with mild attenuated arterioles and an unremarkable macula (figure 6). The left eye has an average-to-small optic nerve with a cup-to-disc ratio of 0.2 x 0.2. All other retinal findings in the left eye are unremarkable.
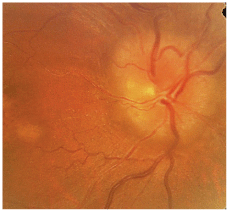
6. A dilated fundus exam in this patient with nonarteritic ischemic optic neuropathy illustrated a hyperemic, edematous optic nerve in the right eye, with mild attenuated arterioles and an unremarkable macula.
A Humphrey 24-2 SITA Standard visual field shows an inferior altitudinal defect O.D. and full field O.S. (figures 7 and 8). On further questioning, the patient denied using any additional medications, including erectile dysfunction drugs.
Diagnosis. The patient has a typical non-arteritic anterior ischemic optic neuropathy (NAION) with a history of vascular disease.
Treatment and follow-up. We informed the patient that no treatment is available for this condition. We started him prophylactically on 81mg of aspirin and warned him not to use erectile dysfunction medications because these could cause a similar event in the other eye.
We explained that vision in his right eye may not improve and that he has about a 20% risk of the same event occurring in the left eye. We instructed him to return in one week for follow-up. Vision at follow-up was unchanged.
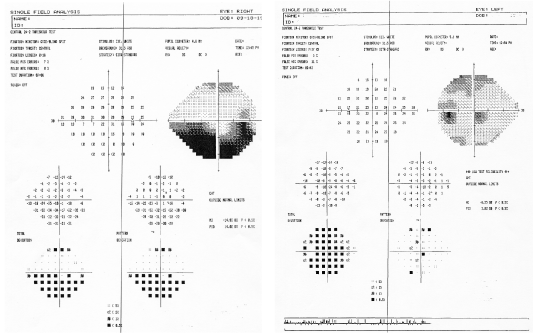
7, 8. A Humphrey 24-2 SITA Standard visual field shows an inferior altitudinal defect O.D. (left) and full field O.S.
Discussion. Afferent pupillary defects are sensitive to optic nerve pathology. We diagnosed the patients condition based on the clinical exam findings, including the cup-to-disc ratio, visual fields and history of vascular disease.
In the absence of vascular risk factors, or in patients younger than 40, infectious, inflammatory, infiltrative or medication-induced optic neuropathy must always be ruled out. For instance, optic neuropathy induced by Viagra (sildenafil, Pfizer) is well documented.14 Additionally, amiodarone, a medication used to manage cardiac arrhythmia, has also been implicated.15,16
You must rule out GCA in patients older than 56. This requires emergent sedimentation rate and C-reactive protein tests. Optic neuritis, which is also an acute event, is usually retrobulbar, and more commonly occurs in patients younger than 50; patients mostly present with pain on eye movements. So, any patients who presents with optic nerve disease that cannot be attributable to typical NAION must be worked up and imaged urgently.
When a patient presents with a relative afferent pupillary defect, a correct differential diagnosis of your clinical signs and symptoms can help steer you toward a timely and appropriate referral. The key is not to panic. Let a case history, examination and ancillary testing help you determine how to handle a patient who presents with an APD.
Dr. Canellos practices with the New York Harbor VA Health Care System at the Brooklyn and
1. Weinstein JM. The pupil. Available at: www.ophthalmic.hyperguides.com/tutorials/neuro/ pupil/default.asp (Accessed April 10, 2008).
2. Duong DK, Leo MM, Mitchell EL. Neuro-ophthalmology. Emerg Med Clin N Am 2008 Feb;26(1):137-80, vii.
3. Jun W. Pupil anomalies: reaction and red flags. Available at: http://opt.pacificu.edu/ce/
catalog/pupil_anomalies/index.html (Accessed March 17, 2008).
4. Chen CJ, Scheufele M, Sheth M, et al. Isolated relative pupillary defect secondary to contralateral midbrain compression. Arch Neurol 2004 Sep:61(9):1451-3.
5. Albert DM, Jakobiec FA. Principles and Practice of Ophthalmology. 2nd ed. Vol.5.
6. Kardon R,
7. Hwang JM, Kim C, Kim JY. Relative afferent pupillary defect in patients with asymmetric cataracts. J Cataract Refract Surg 2004 Jan;30(1):132-6.
8. Wilhelm H, Peters T, Ldtke H, Wilhelm B. The prevalence of relative pupillary defects in normal subjects. J Neuroophthalmol 2007 Dec;27(4):263-7.
9. Sankar PS, Pasquale LR, Grosskreutz CL. Uveal effusion and secondary angle-closure glaucoma associated with topiramate use. Arch Ophthalmol 2001 Aug;119(8):1210-1.
10. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology 1980 Jan;87(1):75-8.
11. Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: Validity and reliability of various diagnostic criteria. Am J Ophthalmol 1997 Mar;123(3):285-96.
12. Valle JN, Paques M, Aymard A, et al. Combined central retinal artery and venous obstruction: emergency ophthalmic arterial fibrinolysis. Radiology 2002 May;223(2):351-9.
13. Schmidt DP, Schulte-Monting J, Schumacher M. Prognosis of central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment. AJNR Am J Neuroradiol 2002 Sep;23(8):1301-7.
14. Fraunfelder FW, Pomeranz HD, Egan RA. Nonarteritic anterior ischemic optic neuropathy and sildenafil. Arch Ophthalmol 2006 May;124(5):733-4.
15. Jafari-Fesharaki M, Scheinman MM. Adverse effects of amiodarone. Pacing Clin Electrophysiol 1998 Jan;21(1 Pt 1):108-20.
16. Macaluso DC, Shults WT, Fraunfelder FT. Features of amiodarone-induced optic neuropathy. Am J Ophthalmol. 1999 May;127(5):610-2.

