A 55-year-old white female presented in August 2004 with a bump on the nasal aspect of her right lower lid. The nodule was not tender, she said, but it had been enlarging somewhat since it appeared three months prior. She said the cyst was occasionally itchy, especially while she worked on the computer. She reported no discharge, and the globe was uninvolved. She had been a patient of mine several years ago, but had not been in for an appointment since 1991.
Her current medications included Zoloft (sertraline, Pfizer), Synthroid (levothyroxine, Abbott), and metoprolol. She reported allergies to penicillin and sulfa-based antibiotics, and GI toxicity to codeine.
Diagnostic Data
Uncorrected entering acuity was 20/70 O.D. and 20/200 O.S. Best-corrected acuity through hyperopic astigmatic correction was 20/25 O.U. Pupils were equal, round and reactive to light and accommodation, with no afferent pupillary defect. Extraocular motilities were full in all diagnostic positions of gaze. The patient demonstrated no evidence of orbitopathy or lid abnormalities associated with thyroid eye disease. Exophthalmometry was not performed.
Slit lamp examination of the anterior segments demonstrated a sebaceous cyst anterior to the punctal aperture on the right lower lid. Her corneas were clear, and the anterior chambers were deep and quiet O.U.
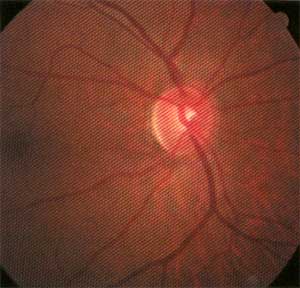
Applanation tensions were 22mm Hg O.D. and 20mm Hg O.S. A non-dilated view of the fundi demonstrated cup-to-disc ratios of 0.50 x 0.70 O.D. and 0.40 x 0.50 O.S. The neuroretinal rim of the right eye was significantly thinned inferiorly, consistent with glaucomatous optic neuropathy. The left neuroretinal rim appeared healthy, with some vertical elongation of the cup in a round optic nerve. Both maculae were clear.
I educated the patient on the nature and management of the sebaceous cyst. I also informed her about the probability of glaucomatous damage O.D. with associated risk factors O.S. We scheduled the patient for a complete glaucoma workup and cyst drainage.
Two weeks later, the patient presented for the glaucoma evaluation and dilated examination. At this visit, applanation tensions were 29mm Hg O.D. and 25mm Hg O.S. Pachymetry readings were 566m O.D. and 559m O.S. Threshold white-on-white visual fields demonstrated a superior arcuate paracentral scotoma O.D. with early nasal step formation, and paracentral arcuate depressions superiorly O.S. Reliability indices were high.
Gonioscopy demonstrated grade IV open angles O.U., with normal to minimal trabecular pigmentation.
On dilation, close examination of the neuroretinal rim O.D. confirmed my initial suspicions: The inferior rim at 6 oclock demonstrated excavation and substantial rim tissue loss. The left optic nerve demonstrated a very small Drance hemorrhage at the 5 oclock position. While the rim was more full in the left eye than the right, it was still suspicious for evidence of previous damage.
Should this patient initiate medication immediately in both eyes, or should we try a monocular trial first?
Discussion
This patient clearly has open-angle glaucoma that is greater in the right eye than in the left. Visual field studies correspond to neuroretinal rim appearance and IOP asymmetry. Given the level of the IOP readings, as well as the difference in IOP readings between the two visits, I would further classify the glaucoma as pressure-dependant glaucoma.
This patient obviously requires intervention to stem the damage. The Collaborative Initial Glaucoma Treatment Study has shown that primary surgical intervention (trabeculectomy) or medical intervention are equally effective in reducing the progression of visual field loss in pressure-dependant glaucoma patients.1 Quality-of-life assessments in both groups appear similar overall. In the United States, however, topical medications have been the typical first-line therapy.
How would you handle this patient? If you believe that she is best managed with topical therapy, one of the initial questions you must answerbeyond which medication to initiateis whether you plan to do a monocular trial. Historically, I have not done monocular trials, as I felt it was best to evaluate each eye individually. But that was a matter of personal preference, without firm data to back up that decision.
A recent study has now given us concrete data that helps us decide whether to employ a monocular trial.2 The general premise of a monocular trial is to assess the efficacy and safety profile of a topical glaucoma medication in one eye before using it in the fellow eye. The assumption: A good response in the treated eye should result in a comparable result in the fellow eye. Likewise, an untoward event after initiation of the medication in one eye would preclude initiation of therapy in the fellow eye.
Beta-blockers, though, have significant crossover effect (due to systemic absorption) in the non-treated fellow eye, and absolute measures of IOP reduction in the fellow eye, once treated, show a reduced effect.3 On the other hand, latanoprost and other prostaglandins are believed to have little carryover effect;4 they are believed to give a more accurate picture of fellow eye effects in monocular trials.
Regardless of the drug, however, recent research indicates that using data from a monocular trial in one eye bears little correlation to pressure reduction in the fellow eye.2 In this study, the main outcome measure was pressure reduction in the fellow eye. Researchers analyzed data from 52 patients, looking at either raw IOP reduction or percentage of IOP reduction in the fellow eye, and found that the initial eye undergoing therapy had little correlative value in determining what would happen to the IOP in the fellow eye.
While beta-blockers were not excluded from the study, a particular subset of patients (26) was treated with latanoprost (which has little carryover effect). The results from this subgroup also confirmed no correlation between responses from the initially treated eye and the fellow eye.
I chose to manage this patient initially with Travatan (travoprost, Alcon), 1 drop in both eyes h.s. While this is how I typically would have managed such a patient, I can now have more assurance in this approach due to this latest research.
Does that mean you should no longer do monocular trials? Perhaps. You may certainly continue to do so if that is your preference. But the study does require you to recognize that the response in the fellow eye will not necessarily be similar to the first eye. And, if youll have to evaluate each eye individually anyway, why bother with a monocular trial in the first place? n
- Feiner L, Piltz-Seymour JR. Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol 2003 Apr;14(2):106-11.
- Realini T, Fechtner RD, Atreides SP, Gollance S. The uniocular drug trial and second-eye response to glaucoma medications. Ophthalmology 2004 Mar;111(3):421-6.
- Piltz J, Gross R, Shin DH, et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the Ocular Hypertension Treatment Study. Am J Ophthalmol 2000 Oct;130(4):441-53.
- Alm A, Stjernschantz J. Effects of intraocular pressure and side effects of 0.005% latanoprost applied once daily evening or morning. A comparison with timolol. Scandinavian Latanoprost Study Group. Ophthalmology 1995 Dec;102(12):1743-52.

