 |
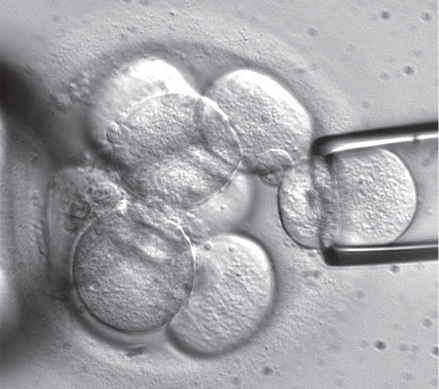
| An embryonic stem cell biopsy in progress. This method acquires human stem cells without destroying embryos.
|
The news was first reported in an article from Reuters, and later confirmed by the company behind the clinical trial, Advanced Cell Technology.
The Phase I/II prospective clinical trial is still underway and otherwise under wraps. The study is investigating transplantation of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells (hESCs) in patients with advanced dry AMD. Two additional clinical trials are also investigating this treatment in patients with Stargardt macular dystrophy.
“The ultimate therapeutic goal will be to treat patients earlier in the disease processes, potentially increasing the likelihood of photoreceptor and central visual rescue,” the investigators wrote in a preliminary report in 2012.1
News of this patient’s dramatic improvement was met with both hopefulness and skepticism by the scientific and ophthalmic communities. The big question: Can this one patient’s exceptional results be replicated in others?
“The devil is in the details,” says Gordon C. Hendricks, OD, MS, of El Paso, Texas, whose own research involved the regenerative properties of retinal stem cells in fish eyes. “This is very promising research. But what makes it difficult to achieve in humans is exogenously creating those stem cells and then delivering them to the human retina.”
1. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012 Feb 25;379(9817):713-20.
|
|

