 Managing inflammation of the cornea and other ocular surface structures can present a challenge to even the most seasoned eye care professionals. While we now have access to many potent topical and systemic anti-inflammatory agents, the circumstances surrounding ocular surface inflammation––as well as the potential complications of pharmaceutical intervention––can present several clinical challenges.
Managing inflammation of the cornea and other ocular surface structures can present a challenge to even the most seasoned eye care professionals. While we now have access to many potent topical and systemic anti-inflammatory agents, the circumstances surrounding ocular surface inflammation––as well as the potential complications of pharmaceutical intervention––can present several clinical challenges.
As we know, inflammation is the body’s attempt at protecting itself by isolating and removing perceived harmful stimuli, including damaged cells, irritants or pathogens. While clinicians view the inflammatory cascade as degradative, in actuality this process is necessary to initiate healing. It is only when inflammation persists after the inciting trauma has been eliminated that detrimental effects ensue.
NSAIDs and Steroids
Usually, we treat inflammation with two broad categories of medications: nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids.
• NSAIDs are used primarily to ameliorate mild to moderate pain, reduce joint inflammation and alleviate fever. These medications interfere with arachidonic acid metabolism, specifically blocking cyclooxygenase enzymes and reducing prostaglandin synthesis.1
The prototypical NSAID is aspirin; others include acetaminophen, ibuprofen, diclofenac and naproxen. Commonly used topical ophthalmic NSAIDs include Acuvail (0.45% ketorolac tromethamine, Allergan), Prolensa (0.07% bromfenac sodium, Bausch + Lomb) and Ilevro (0.3% nepafenac, Alcon).
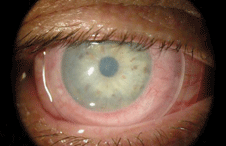
| |
|
A ProKera amniotic membrane graft placed on the ocular surface. Photo: Bio-Tissue
|
• Corticosteroids also interfere with arachidonic acid metabolism but intervene at an earlier point in the pathway, blocking the phospholipase A2 enzyme and imparting anti-inflammatory effects by stabilizing lysosomal membranes, decreasing the release of inflammation-causing lysozymes and decreasing capillary permeability.1 The prototypical corticosteroid is cortisone, but others include prednisone, methylprednisolone, dexamethasone and hydrocortisone. Commonly used topical ophthalmic corticosteroids include Pred-Forte (0.1% prednisolone acetate, Allergan), Lotemax (0.5% loteprednol etabonate, Bausch + Lomb) and Durezol (0.05% difluprednate, Alcon).
Topical corticosteroids generally are indicated to treat inflammation of the anterior segment, including the conjunctiva, cornea, iris and ciliary body. (It is worth noting that Durezol carries a specific indication for the management postoperative inflammation and endogenous uveitis.5)
While the clinical benefits of topical corticosteroids are great, the potential complications are equally well known. Excessive or long-term use of these agents has been associated with ocular hypertension and steroid-induced glaucoma, cataractogenesis, secondary infection and delayed wound healing.
Amniotic Membrane Grafts
The latest approach to ocular inflammation involves the use of amniotic membrane applied to the ocular surface. The human amnion is composed primarily of collagens, proteoglycans, fibronectin and laminin. But perhaps the most important component is hyaluronic acid, which has been shown to suppress T-cell activation, inhibit giant cell formation and promote regenerative healing of damaged tissues.6,7
AmnioGraft (Bio-Tissue), a cryopreserved amniotic membrane allograft for ophthalmic application, was first introduced in the US in 1997 and is still widely used today. AmnioGraft is indicated for the surgical management of numerous conditions, such as pterygium, conjuntivochalasis, corneal defects and chemical burns.
Beyond simply serving as a biological bandage, amniotic membrane possesses unique characteristics, including the ability to promote epithelialization, suppress inflammation, inhibit scarring and angiogenesis, and impart antimicrobial action.8 Moreover, because it is derived from natural human tissue, amniotic membrane has none of the potential adverse effects or complications associated with manufactured, chemically-preserved topical drugs.
In 2005, Bio-Tissue introduced a self-retaining version of cryopreserved amniotic membrane under the trade name ProKera. This device was designed to impart the beneficial aspects of an amniotic membrane graft for treating ocular surface inflammation without the necessity of surgical attachment via sutures or fibrin glue. ProKera’s design incorporates a dual polycarbonate ring system to suspend the membrane and ensure its retention within the ocular fornices, while holding it firmly against the ocular surface.
The introduction of ProKera helped extend the utility of amniotic membrane therapy into primary eye care and optometry. Additionally, it expanded the number and type of conditions that could benefit from such therapy––including neurotrophic keratitis, limbal stem cell deficiency, pseudophakic bullous keratopathy and even infectious keratitis.9-12 Conjunctival complications, such as chemical burns or Stevens-Johnson syndrome, also can be addressed with ProKera because the retention system doubles as a symblepharon ring, preventing conjunctival adhesions while quieting inflammation.
In 2014, Bio-Tissue expanded its product line to include ProKera Slim and ProKera Plus.
ProKera Slim addresses the need for a larger surface area and a thinner, lower-profile retention ring, which purportedly improves comfort for patients with milder forms of ocular surface inflammation.
ProKera Plus incorporates a double layer of amniotic membrane within the original retention ring design, permitting longer duration on the eye and, theoretically, twice the therapeutic capability in cases of more severe inflammatory conditions.
The use of amniotic membrane grafts for ocular surface inflammation likely represents a paradigm shift in thinking for many practitioners. But while topical anti-inflammatory agents still represent the mainstay of therapy for most of the aforementioned conditions, recalcitrant or atypical presentations may require a different approach.
Before considering the use of ProKera, contact your state board of optometry to make certain its use is permitted. Further, while reimbursement for a self-retaining amniotic membrane varies from state to state, most third-party insurers recognize CPT code 65778: “placement of amniotic membrane on the ocular surface, without sutures.”
Dr. Kabat is a paid consultant to Bio-Tissue and Alcon Laboratories. Neither he nor Dr. Sowka has any direct financial interest in any of the products mentioned.
1. Vane J, Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987 Aug;1(2):89-96.
2. Gills JP. Voltaren associated with medication keratitis. J Cataract Refract Surg. 1994 Jan;20(1):110.
3. Shimazaki J, Saito H, Yang HY, et al. Persistent epithelial defect following penetrating keratoplasty: an adverse effect of diclofenac eye drops. Cornea. 1995 Nov;14(6):623-7.
4. Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration and perforation associated with topical non-steroidal ant-inflammatory drugs. Ophthalmology. 2001 May;108(5):936-44.
5. Durezol [package insert]. Alcon Laboratories (A Novartis company). Fort Worth, Texas; 2012.
6. He H, Li W, Tseng DY. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitors. purified from extracts of human amniotic membrane. J Biol Chem. 2009 Jul 24;284(30):20136-46.
7. Hopkinson A, McIntosh RS, Tighe PJ, et al. Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-beta content. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4316-22.
8. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51-77.
9. Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008 May;145(5):787-94.
10. Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008 Aug;126(8):1059-66.
11. Sheha H, Liang L, Li J, Tseng SC. Sutureless amniotic membrane transplantation for severe bacterial keratitis. Cornea. 2009 Dec;28(10):1118-23.
12. Suri K, Kosker M, Raber IM, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013 Sep;39(5):341-7.

