 An elderly woman, over the age of 89, presented to the
William Feinbloom Vision Rehabilitation Center with a report of decreased
vision during the last four weeks. The patient’s daughter reported that her
normally independent mother had become disoriented and fearful during this
period. Our patient reported that it was becoming more difficult to see numbers
on elevators and that she was confused in unfamiliar places.
As a woman who lived alone, she expressed concerns about her
recent loss of independence—as well as the noticeable vision changes.
An elderly woman, over the age of 89, presented to the
William Feinbloom Vision Rehabilitation Center with a report of decreased
vision during the last four weeks. The patient’s daughter reported that her
normally independent mother had become disoriented and fearful during this
period. Our patient reported that it was becoming more difficult to see numbers
on elevators and that she was confused in unfamiliar places.
As a woman who lived alone, she expressed concerns about her
recent loss of independence—as well as the noticeable vision changes.
Patient History
The patient’s systemic history was remarkable for migraine headaches, spinal stenosis, hip and femur surgery, hypertension, and breast cancer. She reported that she had seen her primary care physician two days earlier. After performing a physical examination and ordering a metabolic panel, the physician indicated that all findings were in the normal range.
The patient’s ocular history was remarkable for dry age-related macular degeneration (with the left eye worse than the right). She took Norvasc (amlopidine, Pfizer) for hypertension, Ativan (lorazepam, Biovail Pharmaceuticals) for anxiety and Ocuvite nutraceuticals (Bausch & Lomb). She reported no allergies to medications. She denied any imbalance, weakness, jaw pain, change in appetite or change in frequency or severity of headaches. Throughout the history and testing, our patient appeared to be conscious of time, place and person.
Diagnostic Data
Best-corrected visual acuity measured 20/50 O.D. and 20/120 O.S. The patient’s pupils were equally round and reactive to light, with a trace afferent pupillary defect O.S. Extraocular muscle motilities were full and unrestricted. Confrontation fields and standard Amsler grid testing procedures were unreliable.
During testing of the patient’s left eye, she read letters in the middle of the ETDRS chart and consistently ignored the letters on the left side. The same phenomenon was noted while documenting near acuity with a continuous text chart. She would start in the middle of the sentence and read to the end, omitting the beginning of each sentence on the left side. Throughout testing, she manifested a consistent head turn to the right and she ignored objects and visual targets presented in the left visual field. When the patient was asked a question from a person situated at her left side, she would turn her head away from the questioner and answer with her head turned to the right. When asked to trace the diagonal lines on the Amsler grid, our patient was successful in tracing the lines to the upper and lower right quadrants, but would lose her place when tracing in the left quadrants.
 |
| 1. Goldmann visual field testing revealed bilateral left
hemianopic defects and a shift of visual midline in the patient’s left eye.
|
Diagnosis
We discussed our findings with our patient’s primary care physician and recommended that she be immediately referred for further testing (MRI, carotid Doppler) as well as to a neurologist in order to rule out a CVA or the possibility of brain metastasis secondary to the history of breast cancer. Her physician agreed and provided the necessary referrals. We also requested the results of the comprehensive metabolic panel that had been obtained two days prior. A review of the blood testing revealed that the only out-of-range finding was a slightly elevated alkaline phosphatase level. All other findings were within normal ranges.
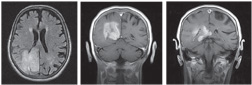 |
| 2. MRI revealed a large neoplasm surrounded by edema that
involved the right posterior parietal, temporal and occipital lobes.
|
Following a neurological consultation, it was determined that our patient had a grade IV astrocytoma, or glioblastoma.
Treatment and Follow-Up
The patient was referred for an evaluation by a neurosurgeon to determine a treatment plan. The neurosurgeon indicated that, due to the patient’s age, as well as the size and progressive nature of the tumor, surgical, chemotherapeutic or radiologic intervention would have a low probability of success. These treatment options would also negatively impact on our patient’s quality of life.
So, as is the case with many elderly patients, our patient and her family opted for no treatment in order to maintain the desired quality of life. A palliative course of therapy was initiated to control pain and to minimize depression and agitation. The patient and her family did not feel that she was ready either emotionally or physically for rehabilitative services. Shortly after her diagnosis, our patient was admitted to a hospice facility. Our patient died three months after the initial diagnosis was made.
Discussion
Gliomas are the most common type of brain tumors (approximately 60% of cases).1 They may be further subdivided by histological features and the degree of malignancy into astrocytomas and oligodendrogliomas. One of the most common and malignant of all glial tumors is a grade IV astrocytoma, or glioblastoma multiforme (GBM). These account for 50% to 60% of all astrocytic tumors. These devastating tumors arise from brain cells called astrocytes, and they aggressively grow and divide. Primary GBMs are typically found in adults between the ages of 50 and 70, and affect males slightly more than females (a ratio of three to two).
In cases when the tumors arise without any evidence of lower malignancy precursor lesions, they will present after a brief clinical history, usually less than three months in duration.1 Secondary GBMs are found in younger adults, typically less than 45 years old. They represent a malignant progression from lower-grade astrocytomas (World Health Organization grades II and III), and the rate of progression varies from one to 10 years.1
Tumor Development and Symptoms
Glioblastoma multiformes are found in the subcortical white matter of the cerebral hemispheres, and they often infiltrate into adjacent cortex or white matter pathways. They occur most frequently in the temporal lobe, followed by the parietal, frontal and occipital lobes. The exact cause of GBMs is unknown; however, the high number of genetic changes in glioblastomas suggests that mutations in genetic pathways may be responsible for the tumor.1 For example, loss of heterozygosity on chromosome arm 10q occurs in 60% to 90% of both primary and secondary glioblastomas. Other genetic mutations being investigated are mutations in p53, a tumor suppression gene, MAGE-E1, PDGF-alpha and MMAC1-E1.1-4 Tumors located in the right parietal lobe region typically manifest in a spatial neglect.5 These right-sided lesions commonly result in a left neglect, because the right hemisphere of the brain attends to both the right and left sides of space. (Conversely, the left hemisphere corresponds primarily to the right side.) A large parietal lesion can create both a dense spatial neglect and hemianopia.
It is often difficult to discern one from the other or if a patient is experiencing a combination of both.6 A patient’s symptoms, posture, behavior and clinical testing all provide valuable clues to diagnosis. Individuals with a homonymous hemianopic visual field deficit typically exhibit a head turn toward the area of the visual field deficit. This is an adaptation that enables them to more efficiently use the residual visual field region. Individuals with spatial neglect, however, posturally shift away from the area of the visual field deficit, actually increasing the severity of their visual disability. This results in disorientation, instability, increased mobility-related incidents and possible injury due to any one of these factors.
Our patient reported such issues in her history. Individuals with spatial neglect and hemianopic visual field defects benefit from occupational therapy as well as orientation and mobility services to prevent injury to themselves during activities of daily living and while ambulating in both familiar and unfamiliar environments. Patients may present with motor weakness, headaches or seizures. Symptoms include nausea, personality changes, difficulty concentrating, headaches with increasing frequency and intensity, hemiparesis, aphasia, and visual loss. Elderly patients are more likely to experience such symptoms as confusion, aphasia, memory loss, personality change and focal motor weakness, with confusion and aphasia being the most common presenting symptoms in patients 65 years and older.7 By comparison, the symptoms most encountered in younger patients with GBM are headaches and seizures.
| TREATMENT
STRATEGIES FOR GLIOBLASTOMA MULTIFORMA
| |||
| TREATMENT
|
GOAL | PROCEDURE
|
OUTCOME |
| None
|
Maintain healthy quality of life. | N/A | Prognosis varies depending upon tumor. |
| Surgery
|
Remove as many tumor cells as possible without worsening patient’s condition. | Needle biopsy may be beneficial before tumor removal. MRI after surgery serves as baseline for future treatment comparison. | Varies depending on location and size of tumor that can be safely removed. Risks versus benefits are weighed. |
| Radiation
|
Kill cells left after surgery and put remaining tumor cells into non-dividing state. | Daily treatments for four to six weeks, two to four weeks after tumor removal. | Side effects vary. Well-responding tumors shrink in size; growth of tumor with radiation is not desirable. |
| Chemotherapy | Kill cells remaining after surgery and put remaining tumor cells into non-dividing state. | Generally given after radiation. Patient-by-patient treatment plan pertaining to timing between radiation and chemotherapy. | Upon completion of treatments, patient goes into
observation by neuro-oncologist every two to four months.
|
| Other
|
Temozolamide; approved by FDA in 2005. Randomized trial showed TMZ with radiotherapy treatment of choice for newly diagnosed GBMs.8,9 | N/A | With all treatments, high-grade tumor cells typically grow
again. Treatment is aggressive in order to prevent regrowth for as long as
possible.
|
| Treatment approaches to GBM vary, but prognosis is typically poor.7 | |||
Treatment and Outlook
Once a confirmed diagnosis is made by an imaging study or biopsy, GBMs are generally treated with surgery, and then with radiation and chemotherapy after the tumor is removed.8 Treatment of these tumors is palliative, and the prognosis for patients is poor.8,9 Currently, there is no curative treatment. Patients treated with surgery, chemotherapy and radiation therapy typically die within one year of diagnosis; however, the survival rate is inversely correlated with the patient’s age.1 Those who receive no treatment live an average of three months after diagnosis.1 Therefore, maintaining quality of life is important in patients who cannot be cured of disease.11 Research indicates that GBM is the brain tumor that requires neurosurgical treatment most frequently in adults older than 75 years.1,12 Considering this information and the increase in the elderly population that is expected in the United States, clinicians who work with elderly patients may encounter more cases of GBM in the future. GBMs can present with visual symptoms that may be confused with progressive vision loss due to a known retinal disease, such as AMD. Examination of elderly patients with glioblastoma multiforme is further complicated by the presence of associated confusion, aphasia, memory loss, personality change and focal motor weakness.5
As astute clinicians, we must listen to the patient’s experiences and note significant behavioral changes. It is also crucial to perform the necessary tests that differentiate between a retinal and neuro-ophthalmic basis for progressive vision loss, especially in cases of preexisting retinal diseases. Referral to appropriate specialists is also essential to ensure that there is not an underlying systemic disorder. Quality of life concerns should always be factored into the management plan of all systemic and visual disorders. As this case indicates, vision loss in a patient is not always what it seems.
Dr. Stone is in private practice in Knightdale, N.C. She completed a residency in low vision rehabilitation at the William Feinbloom Rehabilitation Center at the Pennsylvania College of Optometry at Salus University. Dr. Appel is an associate professor at PCO and at the William Feinbloom Vision Rehabilitation Center. She is active in the low vision section of the American Academy of Optometry and is a Diplomate in Low Vision Rehabilitation. Ms. Graboyes is the Coordinator of Social Services at the William Feinbloom Vision Rehabilitation Center of PCO and an associate professor in the Department of Education and Rehabilitation at Salus University.
1. Bruce J, Kennedy B. Glioblastoma Multiforme: EMedicine Oncology. Available at: www.emedicine.com/med/topic2692.htm (accessed November 2007).
2. Stark AM, Witzel P, Strege RJ, et al. P53, mdm2, EGFR and msh2 expression in paired initial and recurrent glioblastoma multiforme. J Neurol Neurosurg Psychiatry. 2003 Jun;74(6):779-83.
3. Sasaki M, Nakahira K, Kawano Y, et al. MAGE-E1, a new member of the melanoma-associated antigen gene family and its expression in human glioma. Cancer Res. 2001 Jun 15;61(12):4809-14.
4. Lokker NA, Sullivan CM, Hollenbach SJ, et al. Platelet derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002 Jul 1;62(13)3729-35.
5. Driver J, Mattingley JB. Parietal neglect and visual awareness. Nature Neuroscience. 1998 May;1(1):17-22.
6. Liu GT, Volpe NJ, Galetta SL. Neuro-Ophthalmology: Diagnosis and Management. Philadelphia: W.B. Saunders Company, 2001.
7. Lowry JK, Synder JJ, Lowry PW. Brain tumors in the elderly: recent trends in a Minnesota cohort study. Arch Neurol. 1998 Jul;55(7):922-8.
8. Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006 Jun;24(16):2563-9.
9. Henson J. Glioblastoma multiforme and anaplastic gliomas: a patient guide. MGH Brain Tumor Center. 2006 Nov 5. Available at: http://brain.mgh.harvard.edu/PatientGuide.htm (accessed November 2007).
10. Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomized controlled trial. Lancet Oncol. 2005 Dec;6(12):937-44.
11. Medical Management of Glioblastoma Multiforme. Neurosurg Focus. 2006;20(4):E6.
12. Kleinschmidt-DeMasters BK, Lillehei KO, Varella-Garcia M. Glioblastomas in the older old. Arch Pathol Lab Med. 2005 May;129(5):624-31.

