 A 69-year-old white male presented in November 2007 for an acute visit. His chief complaint was a pronounced floater in his left eye, which had persisted for three months. He denied any associated photopsia or antecedent trauma. The floater was transient.
A 69-year-old white male presented in November 2007 for an acute visit. His chief complaint was a pronounced floater in his left eye, which had persisted for three months. He denied any associated photopsia or antecedent trauma. The floater was transient.
The patient had no blur at distance or near. His current medications included Lipitor (atorvastatin calcium, Pfizer) and multivitamins. He reported no drug allergies.
Diagnostic Data
Uncorrected visual acuity was 20/80- O.D. and 20/50-2 O.S. Pupils were equal, round and reactive to light and accommodation, with no afferent pupillary defect. Through a refraction of +2.00D0.50D x 090 O.D. and +1.25D0.75D x 090 O.S. he was corrected to 20/25+ O.D., O.S. and O.U. at distance.
Slit lamp examination of the patients anterior segments was unremarkable. Intraocular pressure measured 25mm Hg O.D. and 33mm Hg O.S. at 10:05 a.m. Through dilated pupils, his crystalline lenses were characterized by bilateral nuclear sclerosis, consistent with his best-corrected visual acuity of 20/25+ O.D. and O.S. His optic nerves were of average size, and his cup-to-disc ratio was 0.40 x 0.50 O.D. and 0.90 x 0.90 O.S.
There was a resolving, diffuse disc hemorrhage located inferiorly on the right neuroretinal rim. The left optic nerve was surrounded 360 degrees by a crescent of zone beta peripapillary atrophy. The neuroretinal rim in the left eye was markedly thinned, with erosion to the inferior edge of the optic canal.
The retinal vascular examination was characterized by grade II arteriolarsclerotic retinopathy, which was consistent with his history of hypercholesterolemia. Both maculae were characterized by fine retinal pigment epithelium (RPE) granulation to the central foveal avascular zones, with no drusen or evidence of subretinal neovascular membrane formation.
There was a pronounced posterior vitreous separation O.S., with a comparable Weiss ring overlying the papillomacular bundle. The peripheral retinal exam was largely unremarkable, with 360 degrees of scattered microcystoid degeneration O.U. There were no retinal holes, tears or tractional phenomena O.U.
Given the patients elevated IOP and optic nerve status, we scheduled him for a full glaucoma work-up three days later. At this examination, the patients pachymetry readings were 503m O.D. and 499m O.S. His IOP measured 22mm Hg O.D. and 29mm Hg O.S. Threshold visual fields in the left eye demonstrated a pronounced superior arcuate scotoma, which did not involve fixation with nasal stepping.
Gonioscopy of the anterior chamber angles demonstrated narrowed but open angles (grade I to grade II+) 360 degrees O.U., with no evidence of peripheral anterior synechiae, iris bomb, excessive trabecular pigmentation, or other angle abnormality. We took stereo optic nerve images, as well as a Heidelberg Retina Tomograph-3 (HRT-3) scan.
HRT-3 of the left optic nerve demonstrated significantly reduced neuroretinal rim area and volume. There was significantly reduced retinal nerve fiber layer thickness in all regions except the superior nasal.
The HRT-3 scan of our patients left eye showed that five of the seven regions were outside the normal limits.
Diagnosis
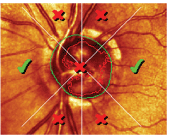
I diagnosed the patient with early open-angle glaucoma O.D. and advanced open-angle glaucoma O.S. We established target IOP at 16mm Hg to 17mm Hg O.D. and less than 12mm Hg O.S. We instructed him to instill one drop of Travatan Z (travoprost, Alcon) O.U. h.s., and to follow-up in two to three weeks.
At the first follow-up, his IOP measured 12mm Hg O.D. and 18mm Hg O.S. While not at target pressure O.S., I decided to continue the Travatan Z O.U. h.s., and bring the patient back for another follow-up in one month for reassessment.
The patient returned on January 8, 2008. His IOP was 14mm Hg O.D. and 19mm Hg O.S., which told me that we were not going to achieve the target IOP in the left eye with monotherapy.
So, I asked the patient to begin using one drop of Combigan (brimonidine tartrate/timolol maleate, Allergan) O.S. b.i.d., after ascertaining that there were no obvious contraindications to beta-blockers.
The day after the follow-up examination, the patient called the office and said that he felt very fatigued, lethargic and lightheaded while golfing. He questioned if the Combigan caused these symptoms, as he had not had any similar experiences before. He had used only two doses of Combigan by the time he called.
The patient returned five days later with IOPs of 17mm Hg O.D. and 19mm Hg O.S. The patients blood pressure was 124/82mm Hg, his respiratory rate was 18 breaths per minute and regular, and his forced expiratory volume was normal. We continued the Travatan, and started him on one drop of Azopt (brinzolamide, Alcon) O.S. b.i.d., and asked him to return for a follow-up in two to three weeks.
Discussion
In this case, a diagnosis of glaucoma was simple and straightforward; it was not complicated or hidden by any ancillary red herring findings. The management strategy for this patient is also relatively straightforward: His IOP needs to be reduced to approximately 16mm Hg O.D. and below 12mm Hg O.S. to lessen the likelihood of further neuroretinal rim loss. We have a good chance of achieving that degree of pressure reduction with topical medications. Our only problem with this patient began when he started Combigan.
Combigan was approved by the FDA in October 2007, and it is typically dosed at one drop b.i.d. Combigan has two well-documented IOP lowering agents: 0.2% brimonidine and 0.5% timolol. Brimonidine is a relatively selective alpha-2 agonist, and timolol is a non-selective beta-blocker that has been used to treat glaucoma since the 1970s.
Was the dizzying experience of the patient on the golf course actually related to Combigan, or was it due to some other event? I believe that the patient did, in fact, experience an adverse reaction to Combigan particularly, to the timolol.
Non-selective beta-blockers, such as timolol, may decrease resting pulse rate and blood pressure and constrict bronchial passageways, among other side effects. The risks for negative effects are increased in patients who take oral beta-blockers, have asthma, or have restrictive pulmonary disease. Also, at-risk patients may experience orthostatic hypotension and/or syncope from using non-selective beta-blockers.
Certainly, the key to avoiding complications and side effects begins with proper patient history and selection. Our patient had no known contraindications to the beta-blocker; yet, he did have a side effect. Perhaps he was overly sensitive to the effects of the 0.5% timolol. Well never know for sure.
But, another plausible reason for this adverse reaction was the actual dosage of the timolol. As previously mentioned, Combigan is intended to be dosed on a b.i.d. basis. Through many years of treating glaucoma with beta-blockers, we have realized that a single dose of 0.5% timolol q.d. is just as effective as a b.i.d. dose. And, for several years now, when we use timolol, levobunolol, or any other topical beta-blockers available in 0.5% strength, we have dosed them on a q.d. basis. The b.i.d. dosing of Combigan may have produced this side effect.
Combigan is a very good fixed combination drug, but we need to be selective in whom we use it. Also, because of the associated side effects of timolol, we must be very careful not to overdose a patient with Combigan. While the drugs side effect profile is well established, it will behoove many readers to be reminded of it here. Some potential side effects of Combigan include: bradycardia, respiratory difficulties and shortness of breath, syncope and behavioral changes and psychic disturbances, including confusion, hallucinations and disorientation.
One study found a high rate of discontinuation (14.3%), due to adverse side effects of fixed combination brimonidine/timolol.1 Interestingly, this same study showed a 30.6% discontinuation rate for brimonidine alone, and a 5% rate for timolol alone.
The early and profound side effects to beta-blocker therapy are easy to recognize. On the other hand, certain side effects may take longer to manifest. Interestingly, I have seen numerous cases in which patients on long-term beta-blocker therapy realize a marked decrease in libido and/or increase in vivid dreams. The onset of these symptoms is often insidious, and it may take years to recognize. So, make sure you query your patients periodically, as their symptoms may not be entirely evident.
When I last saw the patient on January 29, he was medicated with one drop of Travatan Z O.U. h.s. and one drop of Azopt O.S. b.i.d. He was tolerating both medications well, and his IOP measured 14mm Hg O.U. I am still assessing overall diurnal variability in his IOP and considering laser therapy O.S.
1. Sherwood MB, Craven ER, Chou C, et al. Twice-daily 0.2% brimonidine-0.5% timolol fixed-combination therapy vs. monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trial. Arch Ophthalmol 2006 Sep;124(9):1230-8.

